Remedy for acute hepatitis or preventive/remedy for fulminant hepatitis
A technology for acute hepatitis, therapeutic agent, applied in antiviral agents, digestive system, peptide/protein components, etc., can solve problems such as decreased platelet count and poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
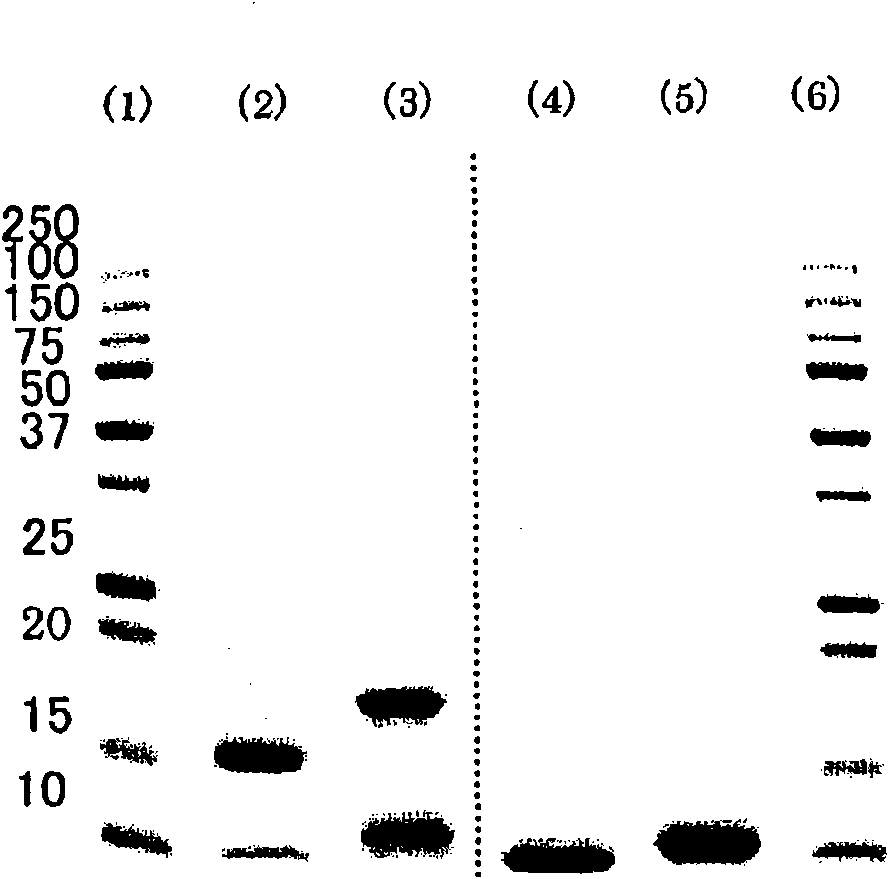
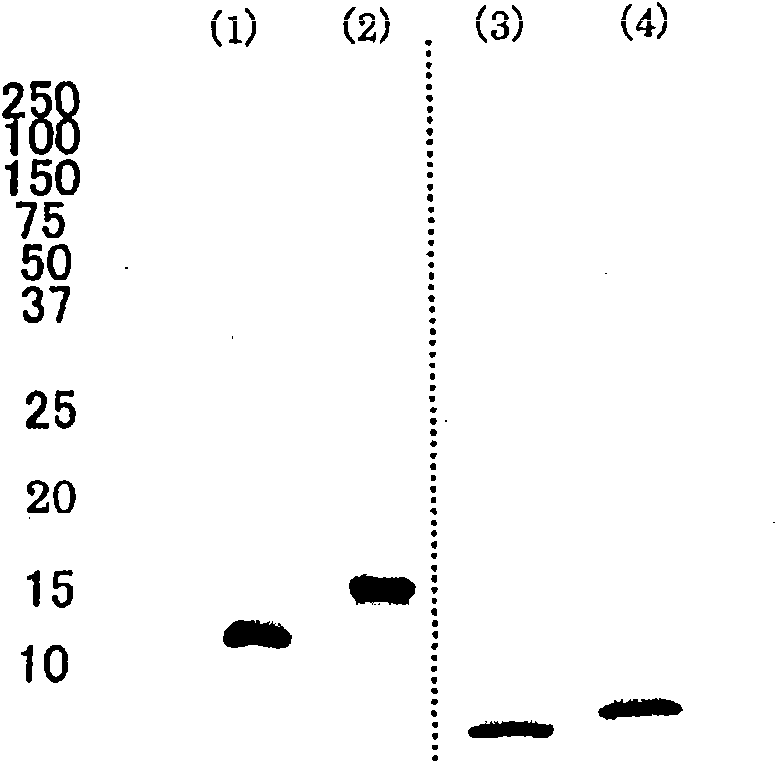
[0046] [0023] Preparation of preparations containing apolipoprotein A-II
[0047] Human plasma from which hepatitis virus and other pathogenic microorganisms have been substantially removed was used as a raw material, and the IV-1 fraction was obtained by Cohn's low-temperature ethanol fractionation of human plasma. 1.2 kg of the fraction IV-1 was dissolved in 2.4 L of a solution containing 100 mM tris(hydroxymethyl)aminomethane and 6 M urea, pH adjusted to 7.8-8.2, in a low temperature room at 2-8°C. Then, an ethanol / chloroform solution (1:1) equal to that of the solution was added and mixed, and centrifuged at 12,000 g for 10 minutes at 4° C. to recover the supernatant protein component. 4.1 L (1.2 times the amount) of ethanol was added to the recovered 3.4 L supernatant, and centrifuged at 12,000 g at 4° C. for 10 minutes to recover 6.8 L of the supernatant. Further, 5.4 L (0.8 times the amount) of ethanol was added to the recovered supernatant, and centrifuged at 12000 g ...
Embodiment 2
[0054] [0025] (1) Construction of recombinant human apolipoprotein A-II expression vector
[0055] The human apolipoprotein A-II gene was cloned by PCR using a human liver cDNA library (manufactured by Takara Biotech Co., Ltd., product code 9505) as a template. Wherein, the sequence shown in SEQ ID NO: 1 in the sequence listing is used as a forward primer, and the sequence shown in SEQ ID NO: 2 in the sequence listing is used as a reverse primer. The obtained PCR fragment was cloned into pCR2.1 vector (manufactured by Invitrogen) according to the TA cloning method using TOPO TA Cloning Kit (manufactured by Invitrogen).
[0056] (2) Preparation of human apolipoprotein A-II expression strain with short bacillus (Bacillus brevis)
[0057] Expression strains were constructed by transforming Bacillus brevis with a vector containing the resulting human apolipoprotein A-II gene. The method for constructing an expression strain was carried out according to the method described in Pa...
example 5
[0058] First construct the plasmid vector according to the method of the patent example 1, and then construct the plasmid vector inserted with the human apolipoprotein A-II gene. Then, according to the method described in Patent Example 5, the brevibacillus H102 (FERM BP-1087) was transformed by electroporation.
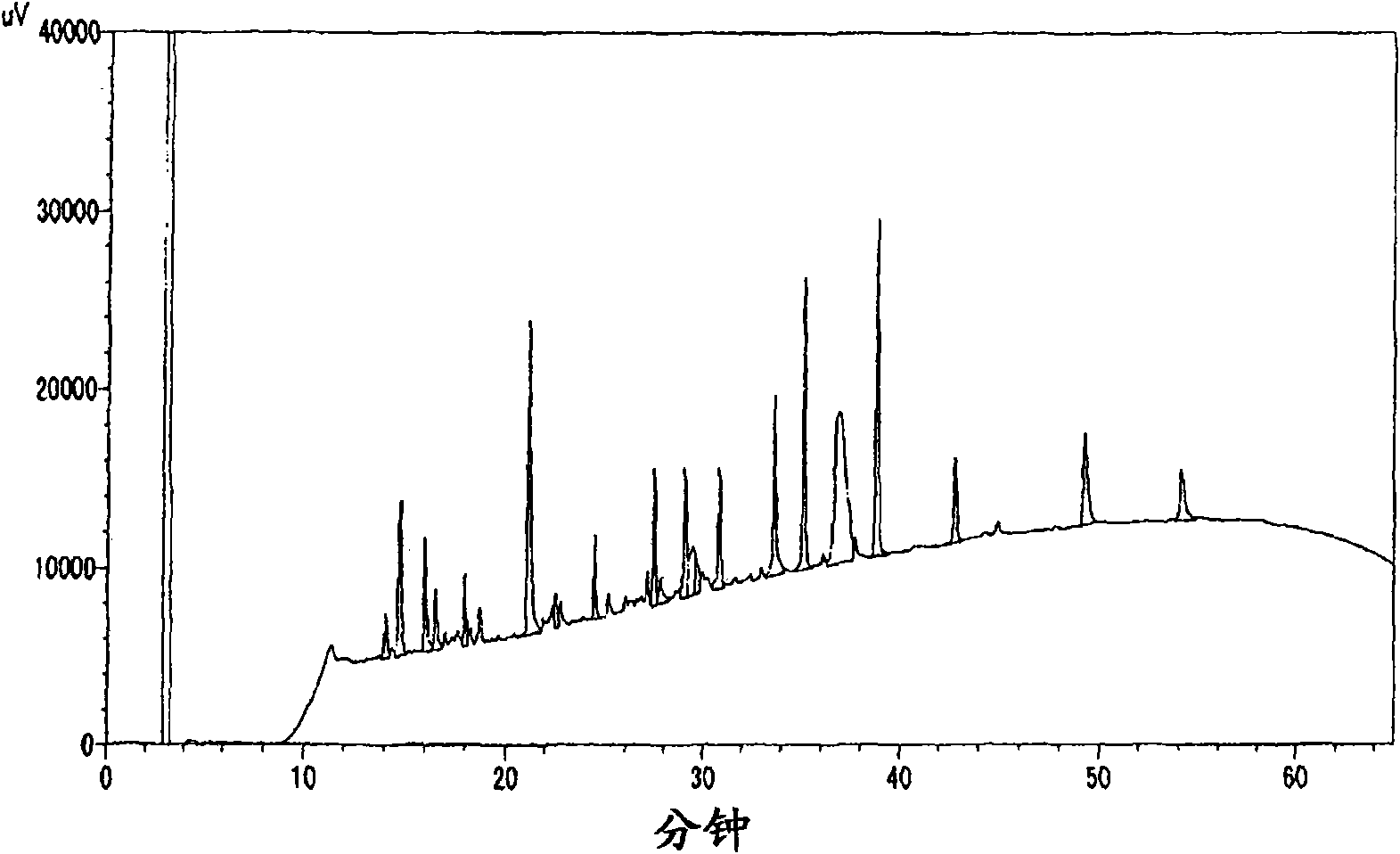
[0059] (3) After the transformant was cultured in TMN medium at 30° C. for 3 days, the culture solution was centrifuged to separate the supernatant to obtain the culture supernatant.
[0060] In order to confirm the amino acid sequence of the gained human apolipoprotein A-II, analyze the base sequence of the pCR2.1 carrier with a 3100 type DNA sequencer (manufactured by ABI company), when it is converted into amino acid, obtain the SEQ ID NO in the sequence listing :3 sequence shown.
[0061] This amino acid sequence is the same sequence as the mature protein part of human apolipoprotein A-II (initial accession number: P02652) described in Swiss-Prot Protein Knowled...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com