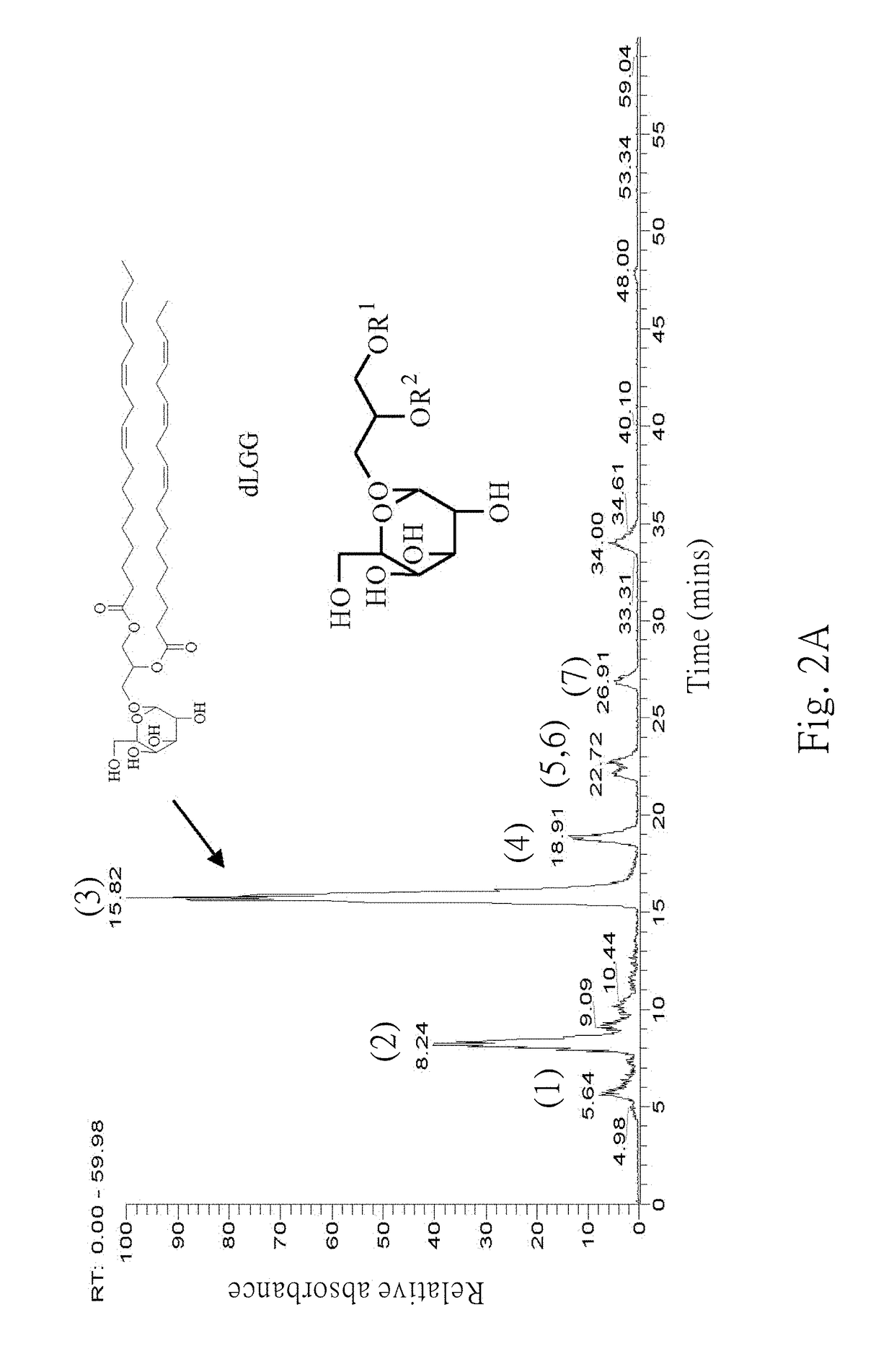
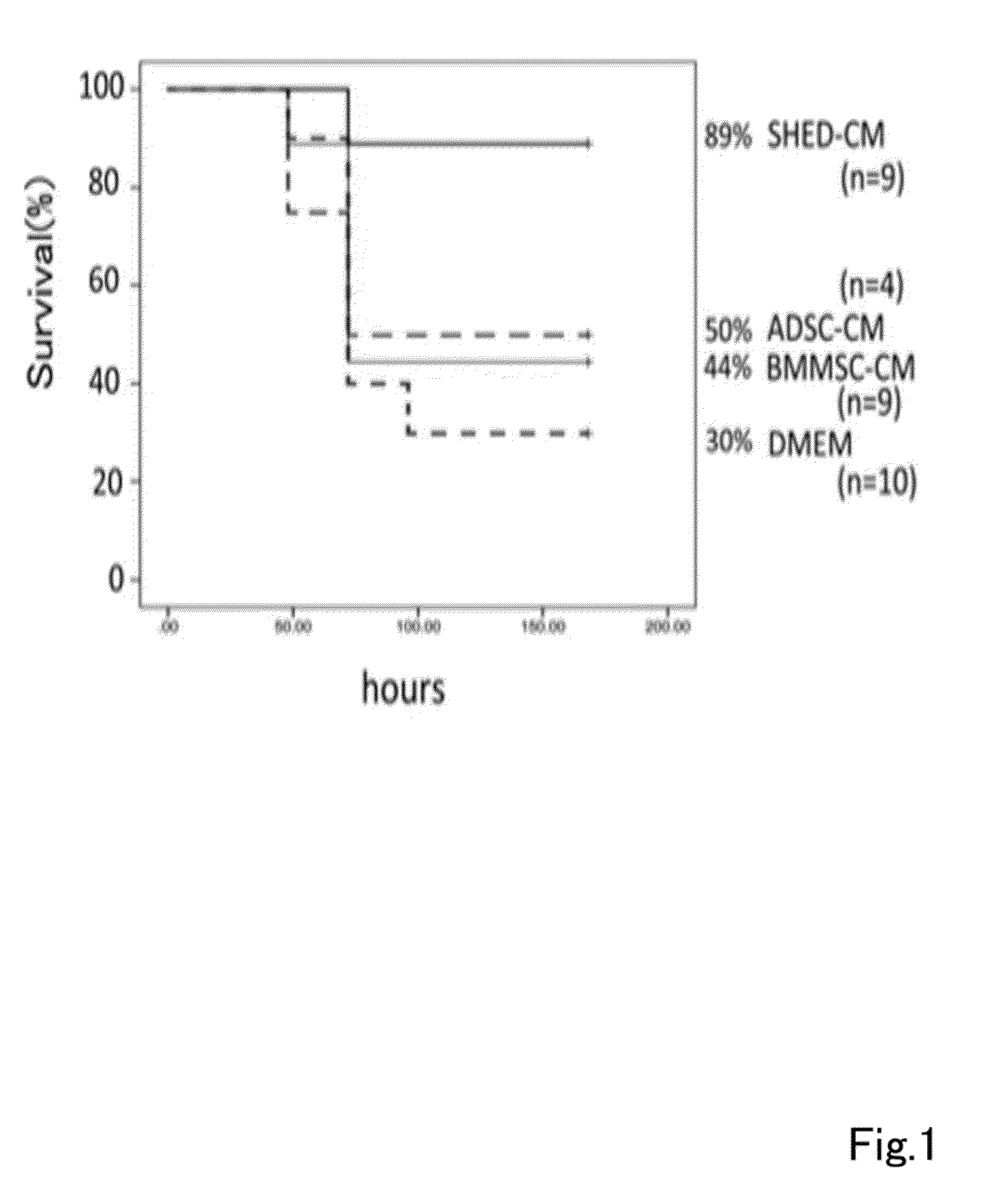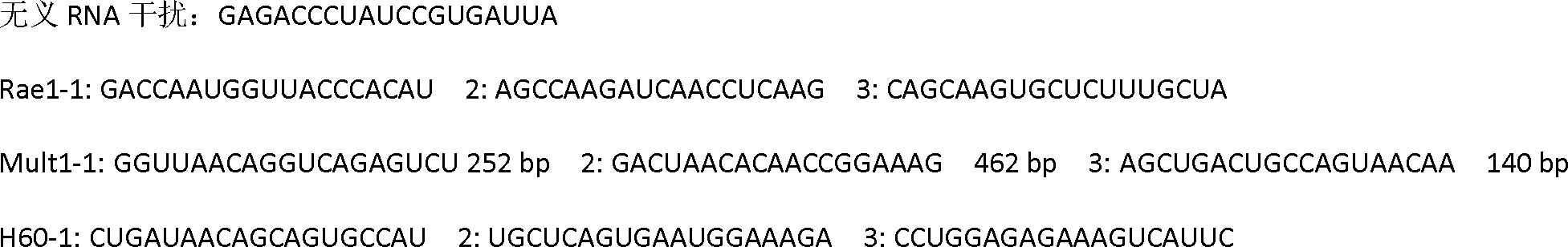Patents
Literature
30 results about "Fulminant hepatitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fulminant hepatitis. a rare and frequently fatal form of acute hepatitis B in which the patient's condition rapidly deteriorates, with hepatic encephalopathy, necrosis of the hepatic parenchyma, coagulopathy, renal failure, and coma.
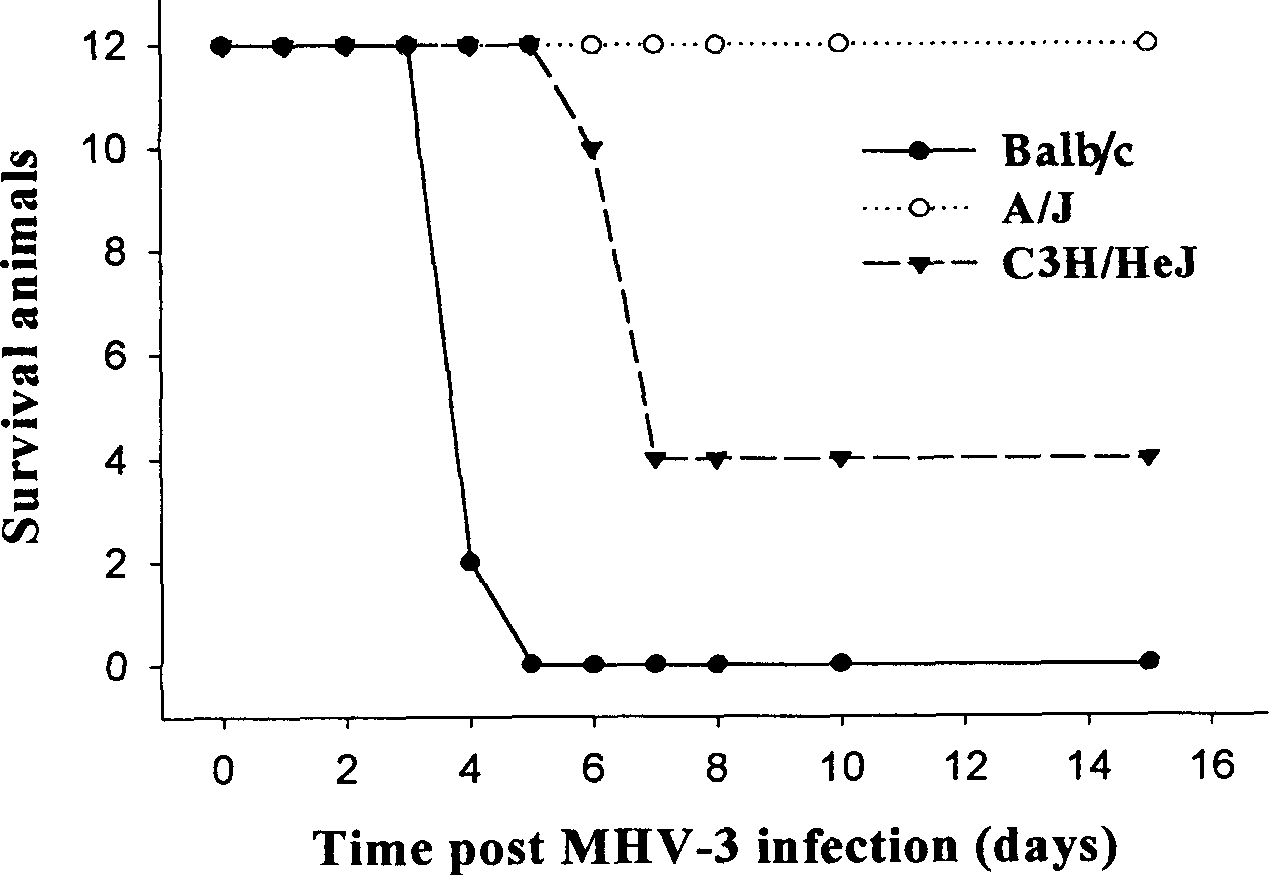
Method for building virus hepatitis mice model
The present invention is the method of establishing mouse model of viral hepatitis. The method includes selection of mouse hepatitis virus-III (MHV-3) of 100-1000 pFu, injecting infection of variousvariety mice of BALB / CJ, C3H / HEJ and A / J mice, and observing the survival rate and liver tissue change. Via the said process, three kinds of viral hepatitis mouse model are established, of fulminanthepatitis, chronic hepatitis and symptom-free mouse separately. The establishment of the model provides powerful facilities for systematic research of viral hepatitis in genetic regulation, molecular mechanism and prevention and treatment.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
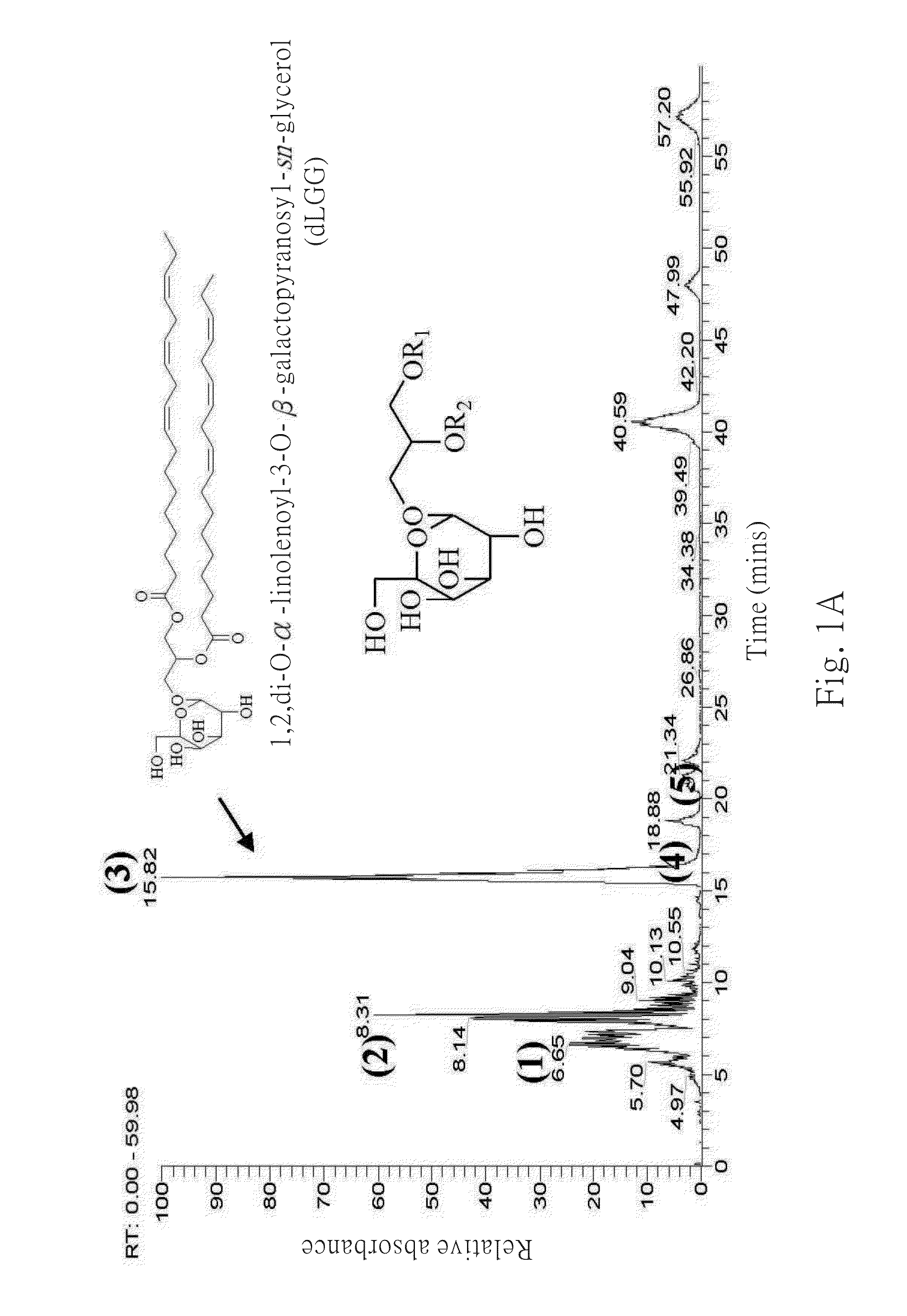
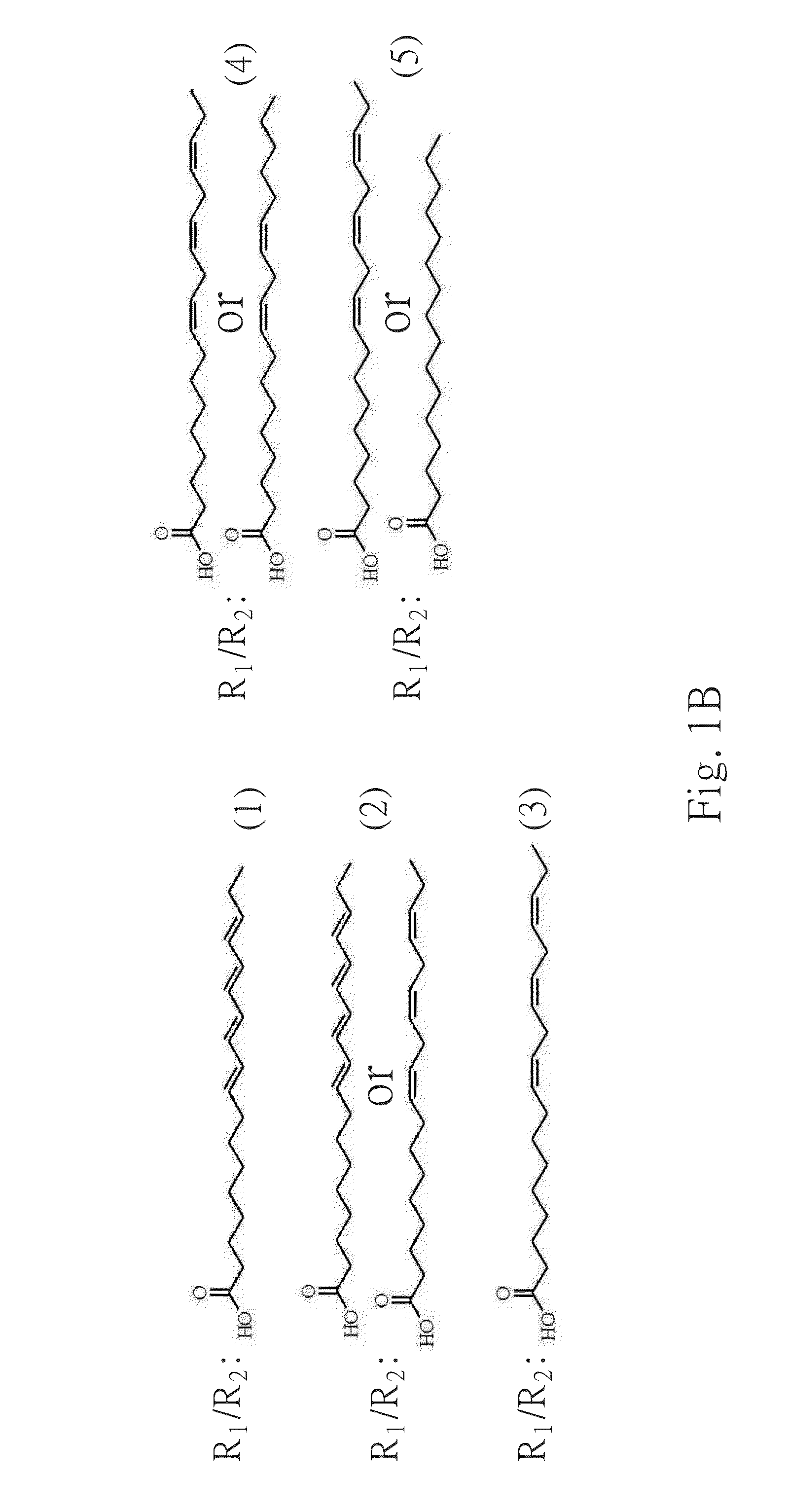
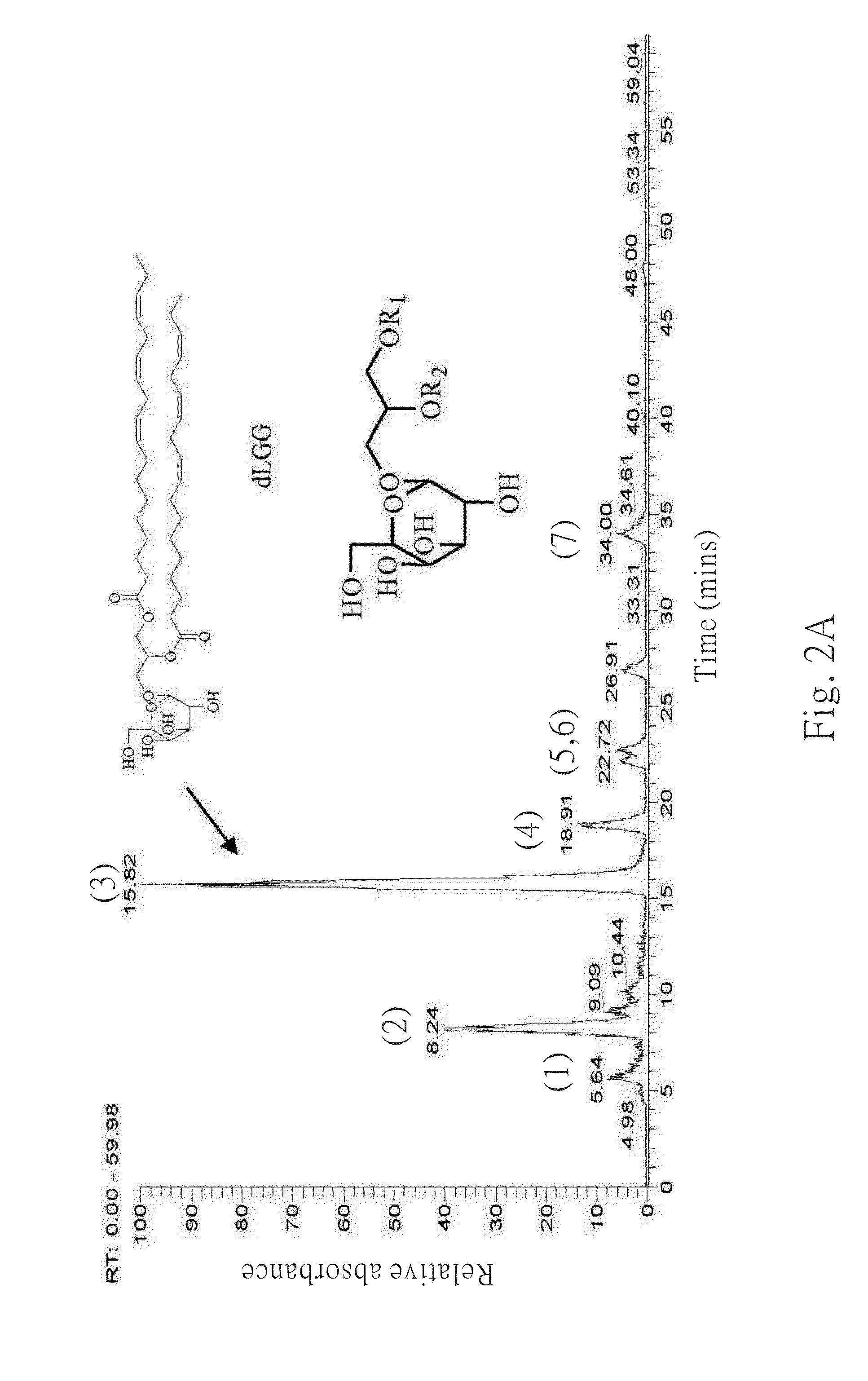
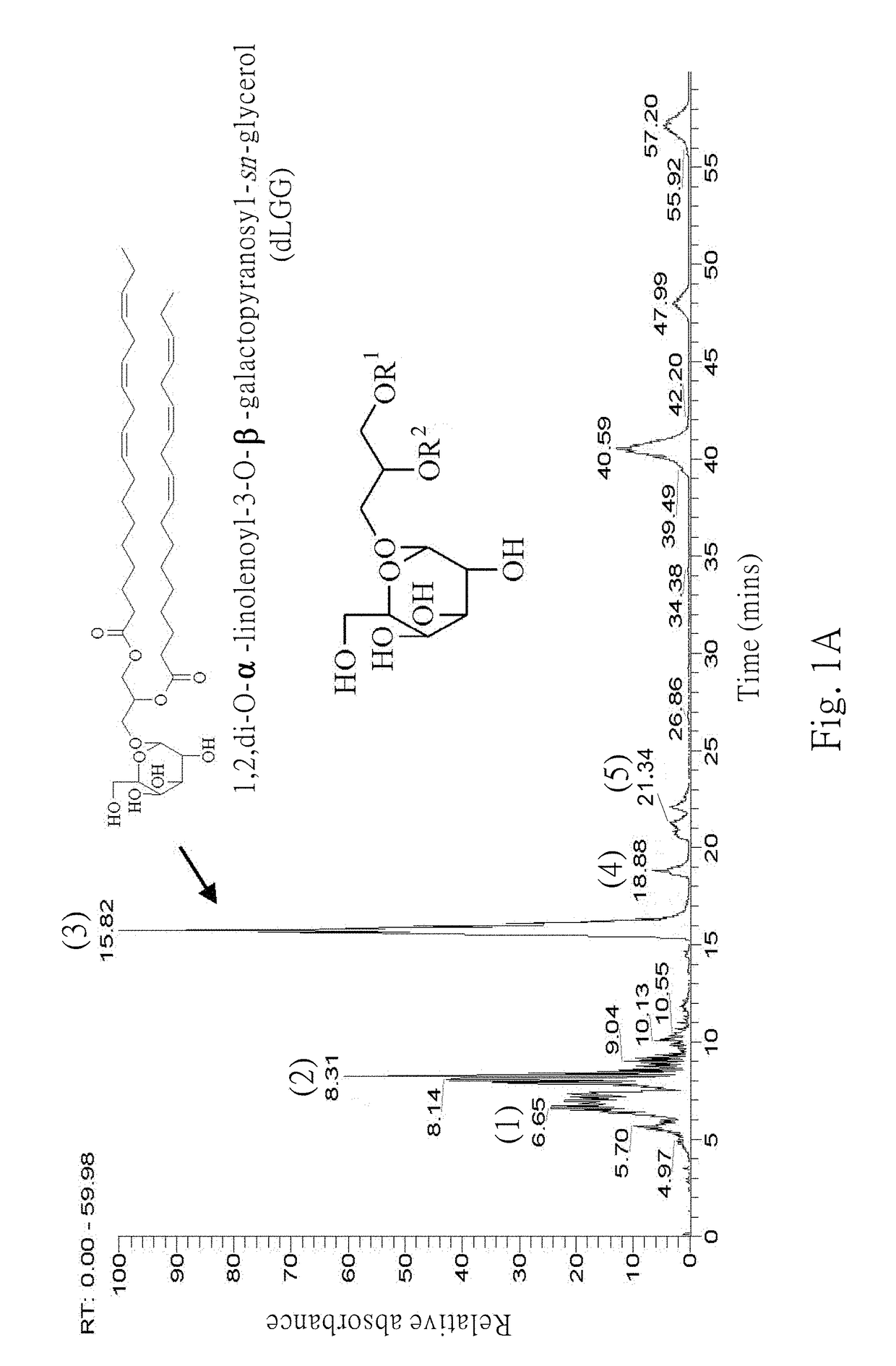
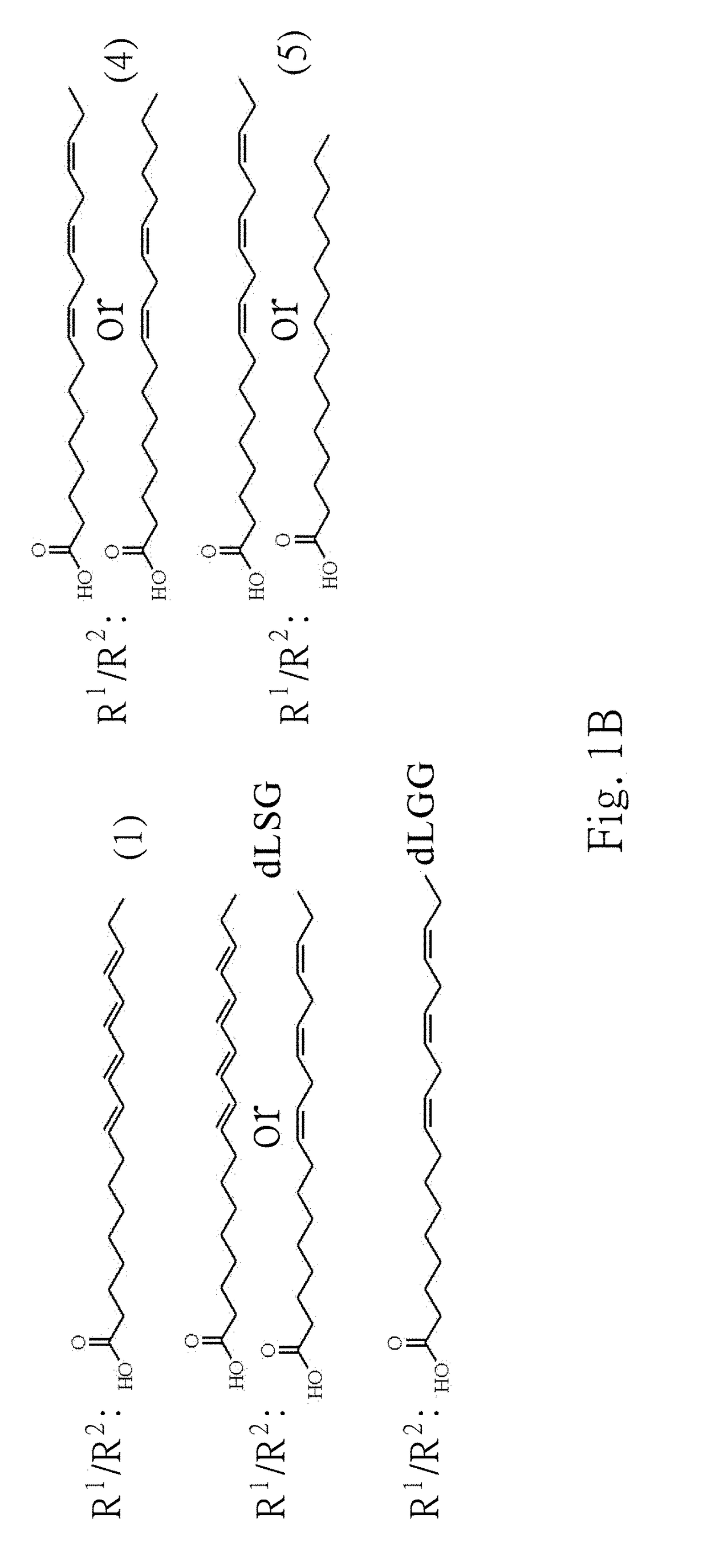
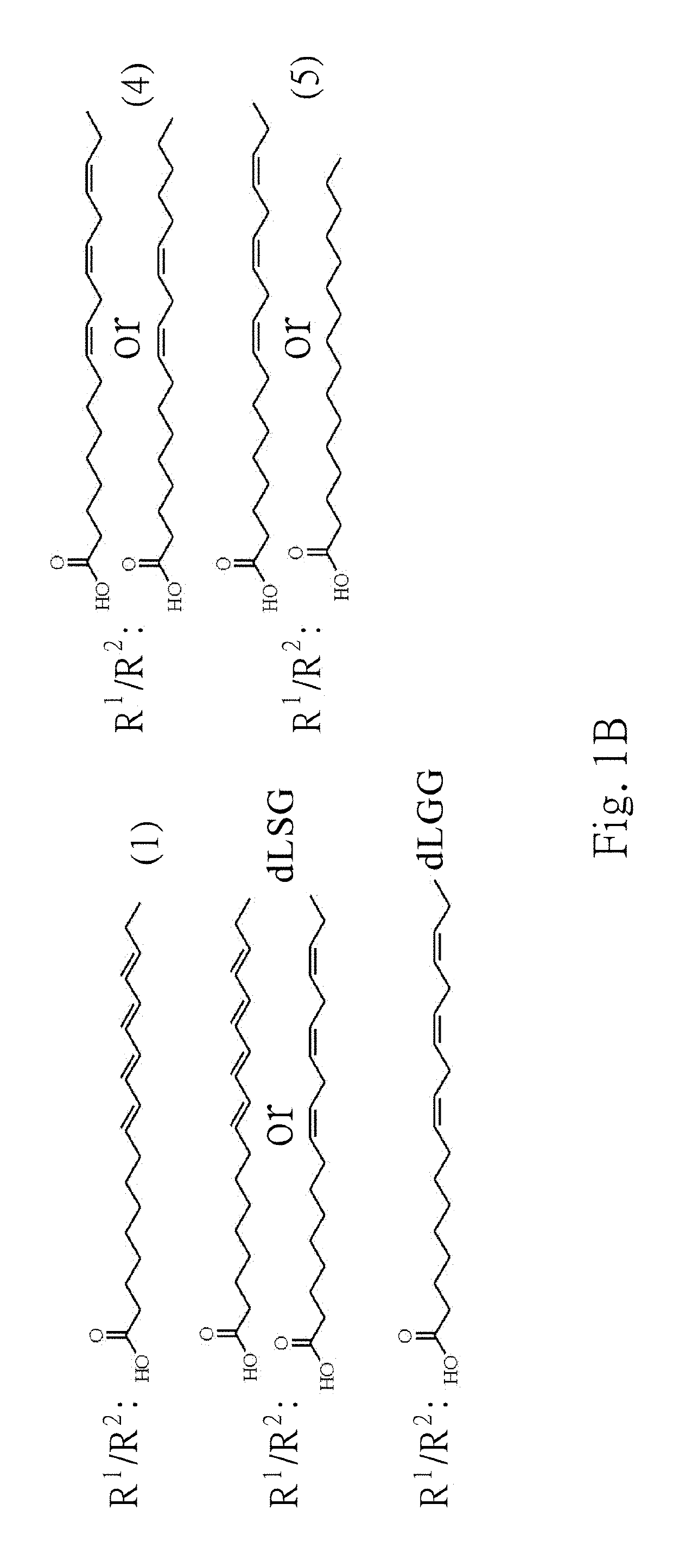
Galactolipids-Enriched Plant Extracts And The Uses Thereof
The present invention is related to a galactolipids-enriched plant extract, prepared by extracting a plant sample selected from a group consisting of: Gynura divaricata subsp. formosana (Asteraceae) (GD), Murdannia bracteata (C. B. Clarke) J. K. Morton ex D. Y. Hong (Commelinaceae) (MB), and Crassocephalum rabens S. Moore (Asteraceae) (CR) with a series of solvents. A pharmaceutical, nutritional, or healthcare composition for protecting or treating acute fulminant hepatitis, for protecting or treating sepsis or related indication thereof, and a composition for skin whitening are also provided herein. These compositions all comprise effective amounts of the galactolipids-enriched plant extracts or purified compounds thereof as bioactive ingredients.
Owner:ACAD SINIC
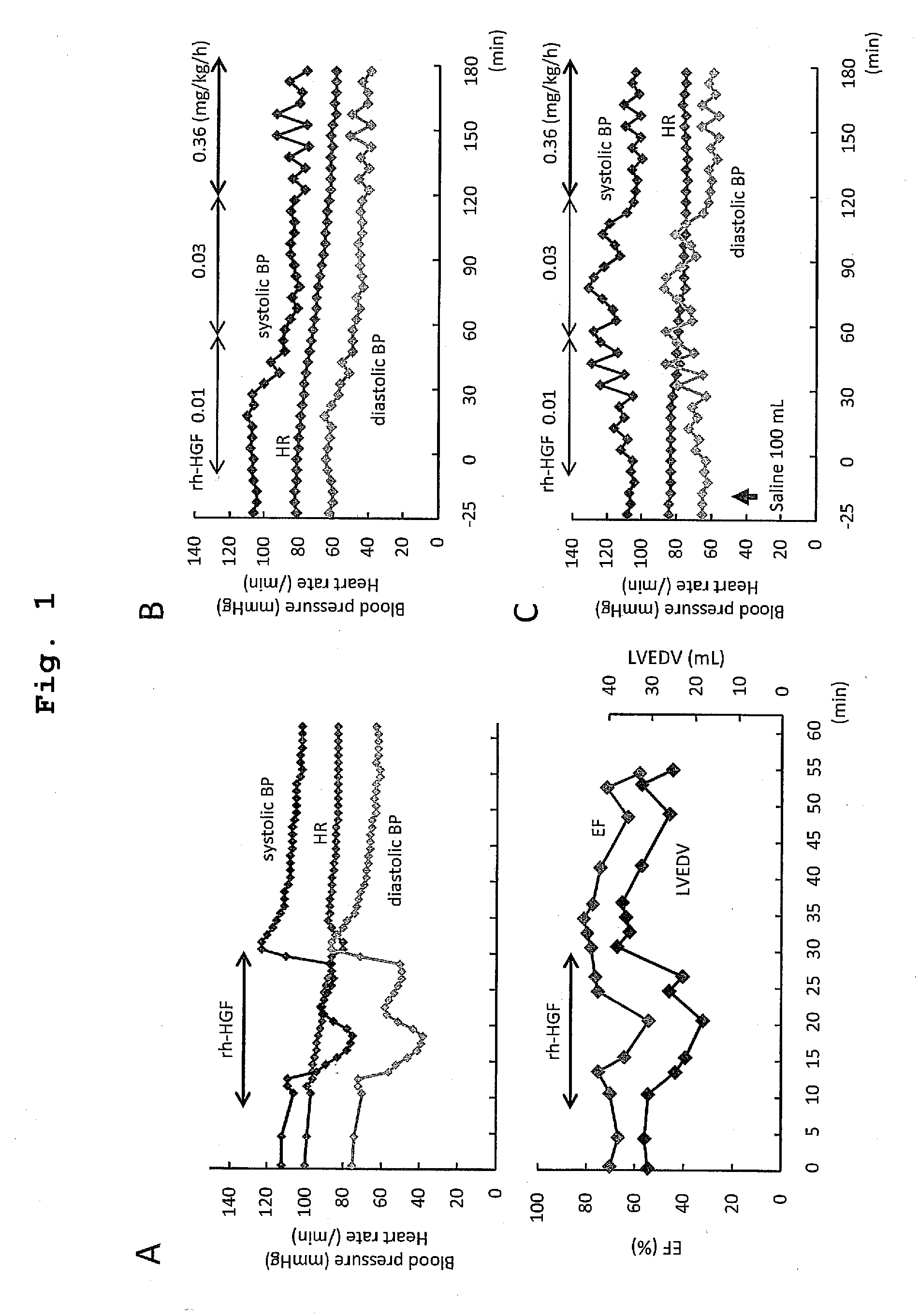
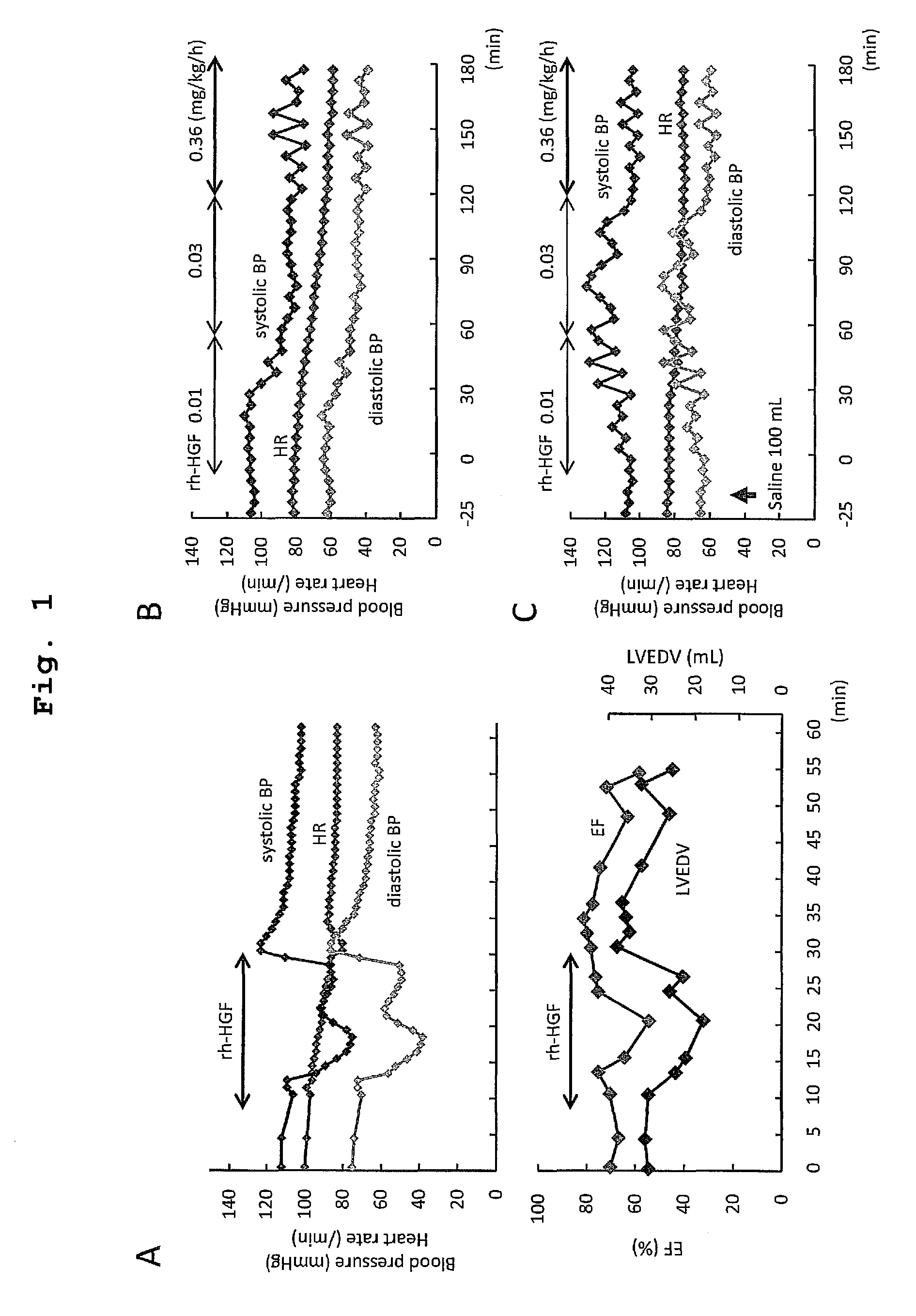
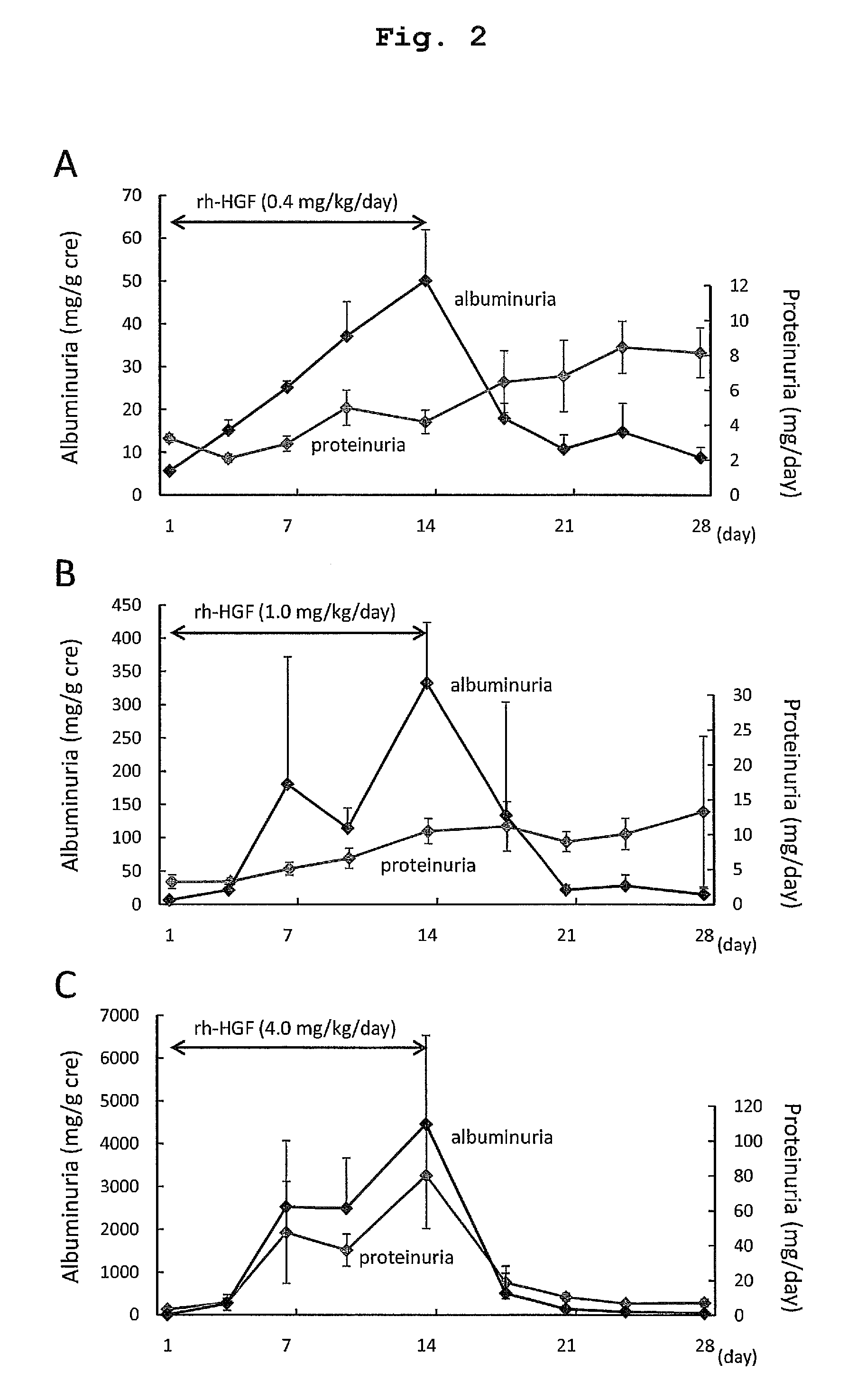
Acute hepatic insufficiency depressant and method for evaluating drug efficacy thereof
ActiveUS20140234341A1Constant effectPoor prognosisPeptide/protein ingredientsHepatocyte-growth/scatter/tumor-cytotoxic factorHepatic comaDepressant
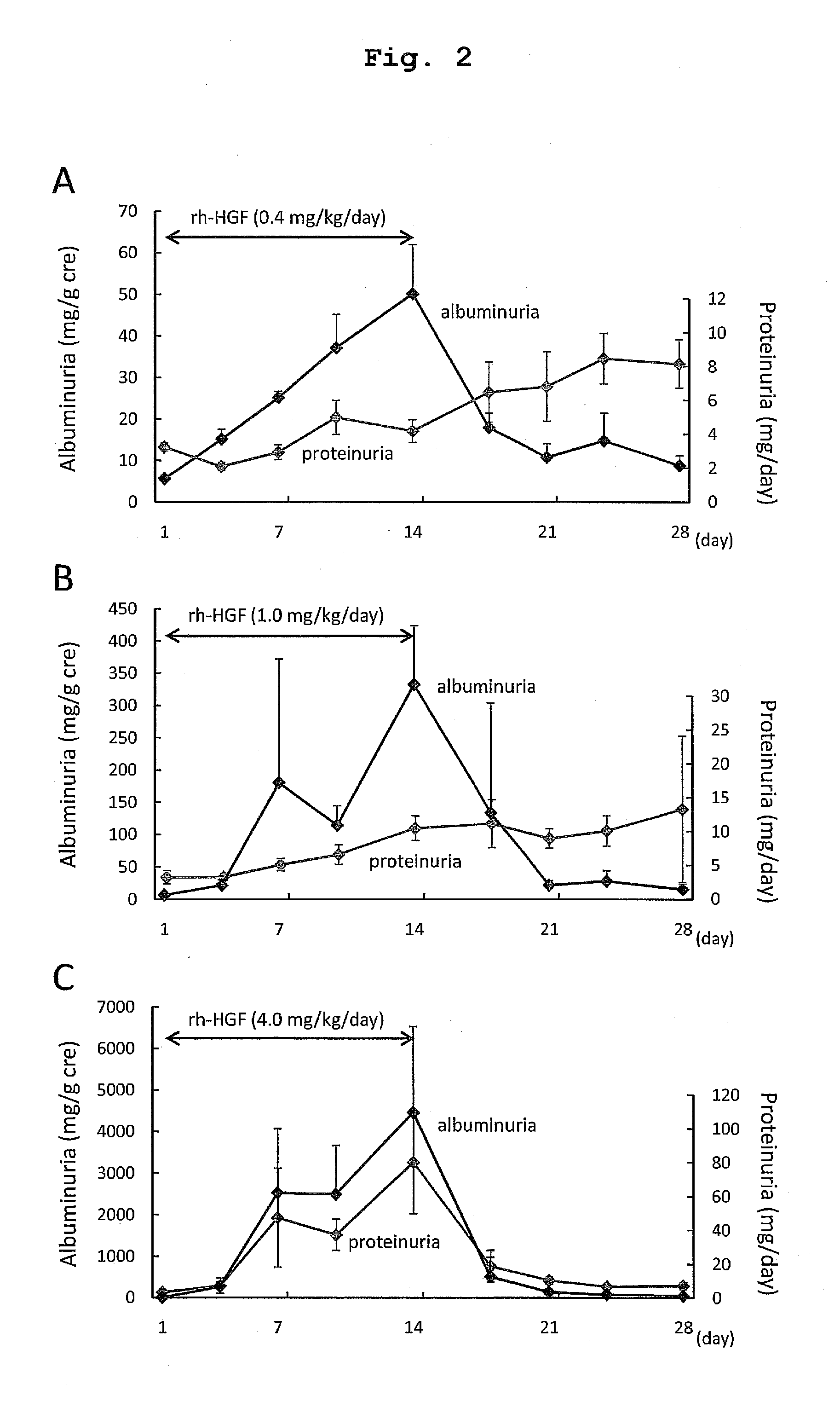
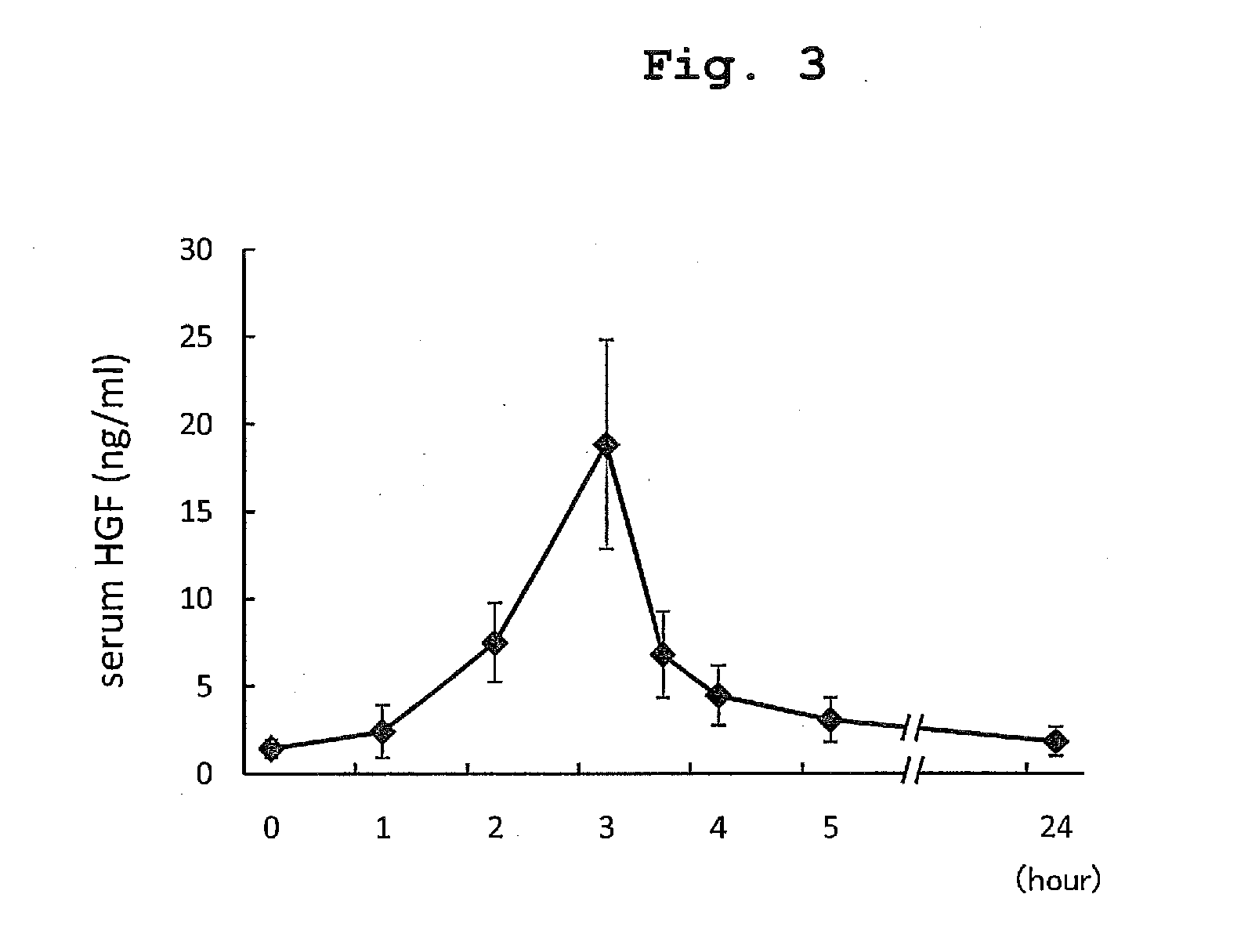
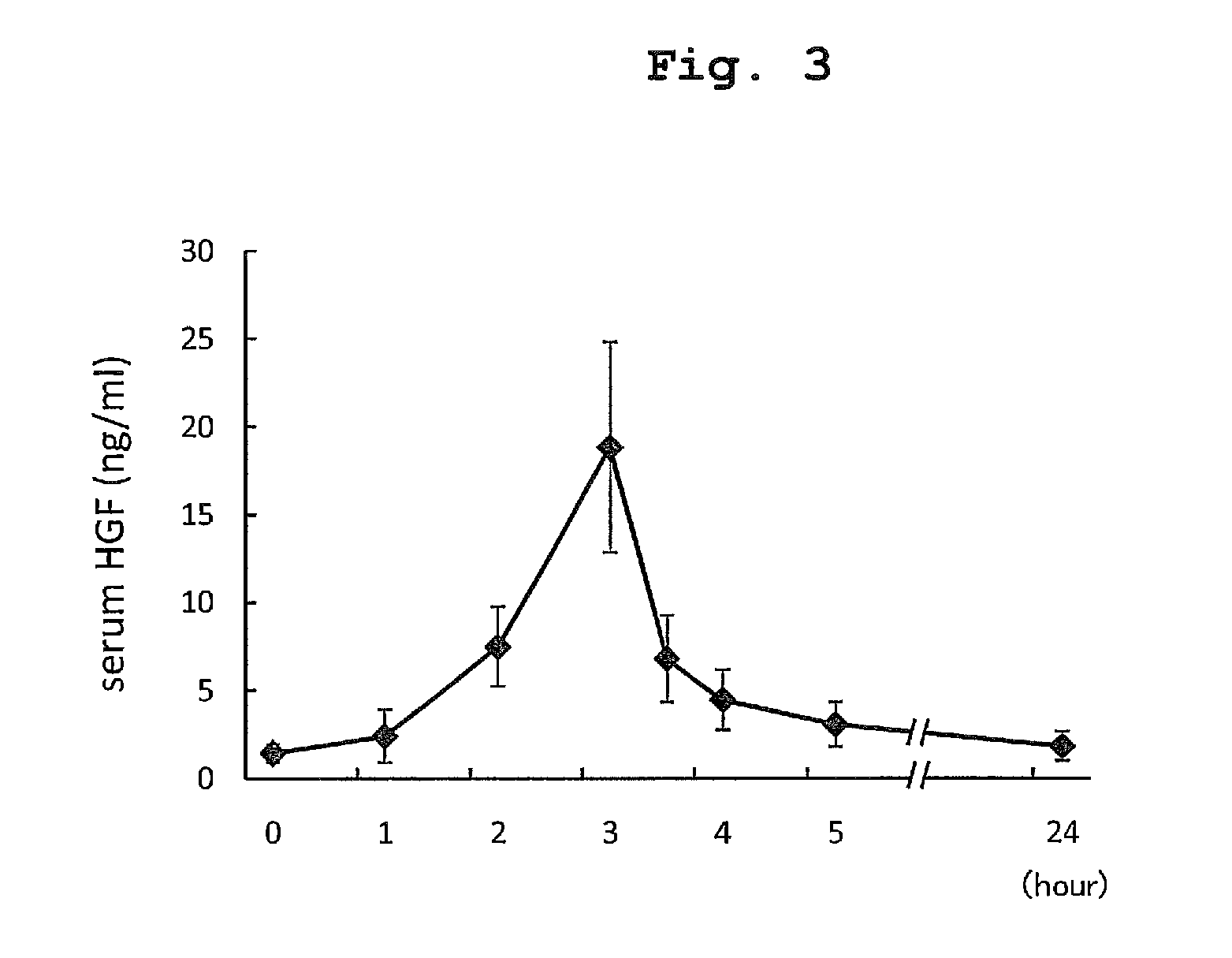
The present invention provides a therapeutic agent for acute liver failure containing a hepatocyte growth factor (HGF), particularly an agent for treating fulminant hepatitis or late onset hepatic failure or suppression of progression of acute liver failure without hepatic coma to fulminant hepatitis or late onset hepatic failure. The present invention also provides a method for evaluating the efficacy of HGF including measuring the amount of α-fetoprotein (liver regeneration biomarker) and / or soluble Fas (anti-apoptotic biomarker) in a sample obtained from a liver injury patient administered with HGF.
Owner:KYOTO UNIV
Galactolipids-enriched plant extracts and the uses thereof
ActiveCN104208114AAvoid damageInhibit expressionAntibacterial agentsCosmetic preparationsAdditive ingredientAsteraceae
The present invention is related to a galactolipids-enriched plant extract, prepared by extracting a plant sample selected from a group consisting of :Gynura divaricata subsp. formosana (Asteraceae) (GD), Murdannia bracteata (C. B. Clarke) J. K. Morton ex D. Y. Hong (Commelinaceae) (MB), and Crassocephalum rabens S. Moore (Asteraceae) (CR) with a series of solvents. A pharmaceutical, nutritional, or healthcare composition for protecting or treating acute fulminant hepatitis, for protecting or treating sepsis or related indication thereof, and a composition for skin whitening are also provided herein. These compositions all comprise effective amounts of the galactolipids-enriched plant extracts or purified compounds thereof as bioactive ingredients.
Owner:ACAD SINIC
Bh4-fused polypeptides
InactiveUS20030152946A1Improve featuresInhibit apoptosisPeptide/protein ingredientsAntibody mimetics/scaffoldsIschemic diseaseSequence identity
A BH4 fusion polypeptide comprising an amino acid sequence of a polypeptide capable of exhibiting uptake action into a cell or a derivative sequence thereof; and an amino acid sequence selected from the group consisting of (A) an amino acid sequence comprising at least the sequence of BH4 domain (SEQ ID NO: 1) of anti-apoptotic Bcl-2 family protein, (B) an amino acid sequence having substitution, deletion or insertion of at least one amino acid residue in the amino acid sequence of SEQ ID NO: 1, and (C) an amino acid sequence having at least 50% sequence identity to the amino acid sequence of SEQ ID NO: 1, wherein the BH4 fusion polypeptide is capable of inhibiting apoptosis; an apoptosis-inhibitor comprising the BH4 fusion polypeptide mentioned above; a method for treating an ischemic disease, characterized by administering the apoptosis-inhibitor mentioned above to a patient with the ischemic disease to inhibit apoptosis, thereby treating the ischemic disease; and use of the BH4 polypeptide mentioned above, for manufacturing a prophylactic or therapeutic agent for an ischemic disease. According to the present invention, the apoptosis can be efficiently suppressed, so that its application as a therapeutic agent for AIDS, neurodegenerative disorders, osteomyelodysplasia, ischemic diseases, infectious multiple organ failure, fulminant hepatitis, diabetes and the like can be expected.
Owner:SHIONOGI & CO LTD
Chinese medicinal composition for treating hepatitis end-stage diseases and preparation method thereof
InactiveCN101732666AImprove self-coordinationFunction increaseDigestive systemMammal material medical ingredientsEnd stage diseaseTherapeutic effect
The invention discloses a new Chinese medicinal composition for treating hepatitis end-stage diseases and a preparation method thereof. The Chinese medicinal composition comprises the following raw materials: dried orange peel, radix curcumae, immature bitter orange, slender dutchmanspipe root, indigowoad leaf, nutgrass galingale rhizome, szechwan chinaberry fruit, combined spicebush root, golden thread, wild chrysanthemum, dandelion, Chinese thorowax root, houttuynia cordata thumb, bezoar, redroot gromwell root, kudzuvine root, raw gypsum, rhizoma anemarrhenae, glabrous greenbrier rhizome, weeping forsythiae capsule, double blossom, mongolian snakegourd root, lightyellow sophora root, rhubarb, Chinese dwarf cherry seed, oriental waterplantain rhizome, longhairy antenoron herb, giant knotweed rhizome, longstamen onion bulb, malt, yanhusuo, peach seed, safflower, leonurus heterophyllus, danshen root and chaff flower root. The Chinese medicinal composition can be prepared into any common oral preparations by a conventional Chinese medicinal preparation method. The Chinese medicinal composition can obviously improve symptoms such as debilitation, appetite reduction, nausea, sickness, hepatauxe and liver damage of people with last-stage hepatitis, and aundice and fever of some patients, can obviously improve symptoms such as gum errhysis, nosebleed, skin ecchymosis, hematochezia, hematemesis, hematuria, purpura occurring in lower limbs and buttocks, bloody pleural effusion or ascites and the like of patients with chronic hepatitis or last-stage fulminant hepatitis, and is characterized by exact clinical effects, obvious treatment effect and quick response. Because medicinal and edible medicaments stipulated by National Formulary are basically adopted for combination, the Chinese medicinal composition has the advantages of low cost, and no toxic or side effect.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Therapeutic agent for acute hepatitis or prophylactic/therapeutic agent for fulminant hepatitis
When acute hepatitis progresses to fulminant hepatitis, a large amount of hepatic cells are rapidly broken, and as a result, the prognosis is seriously worsened. Thus, it is important to prognose the progress of acute hepatitis into fulminant hepatitis at an early stage and quickly start an appropriate treatment therefor. Although the prognosis of progress into fulminant hepatitis becomes possible owing to recent advances in test methods and diagnostic techniques, there has been no appropriate prophylactic / therapeutic agent for fulminant hepatitis. The present invention provides a therapeutic agent for acute hepatitis or a prophylactic / therapeutic agent for fulminant hepatitis with little side effect. The problem of the invention was solved by using a composition containing apolipoprotein A-II.
Owner:CHUGAI PHARMA CO LTD
Composition for preventing or treating inflammatory disease
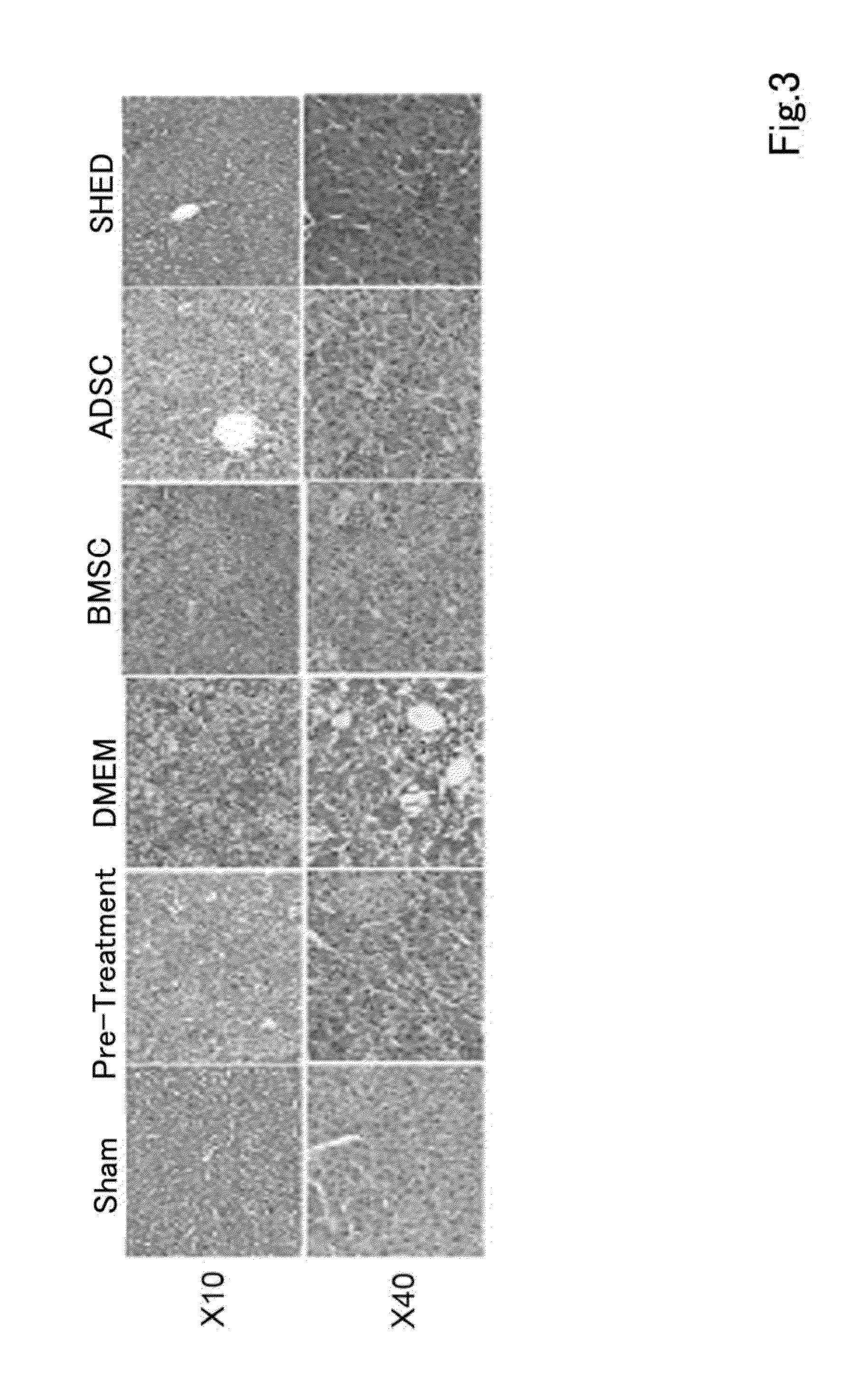
ActiveUS20150366917A1Clinical improvementNervous disorderAntipyreticMedicineInflammatory bowel disease
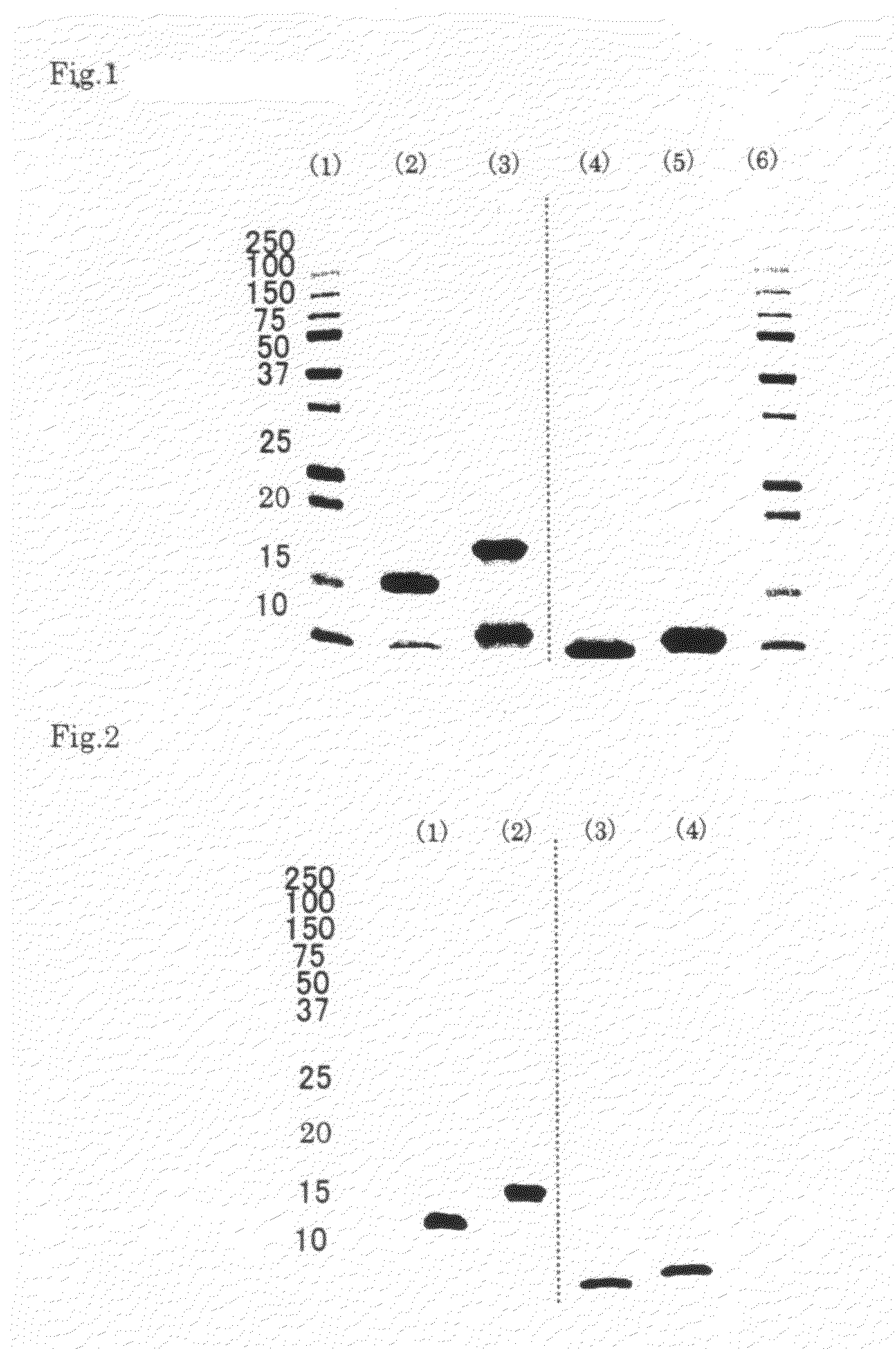
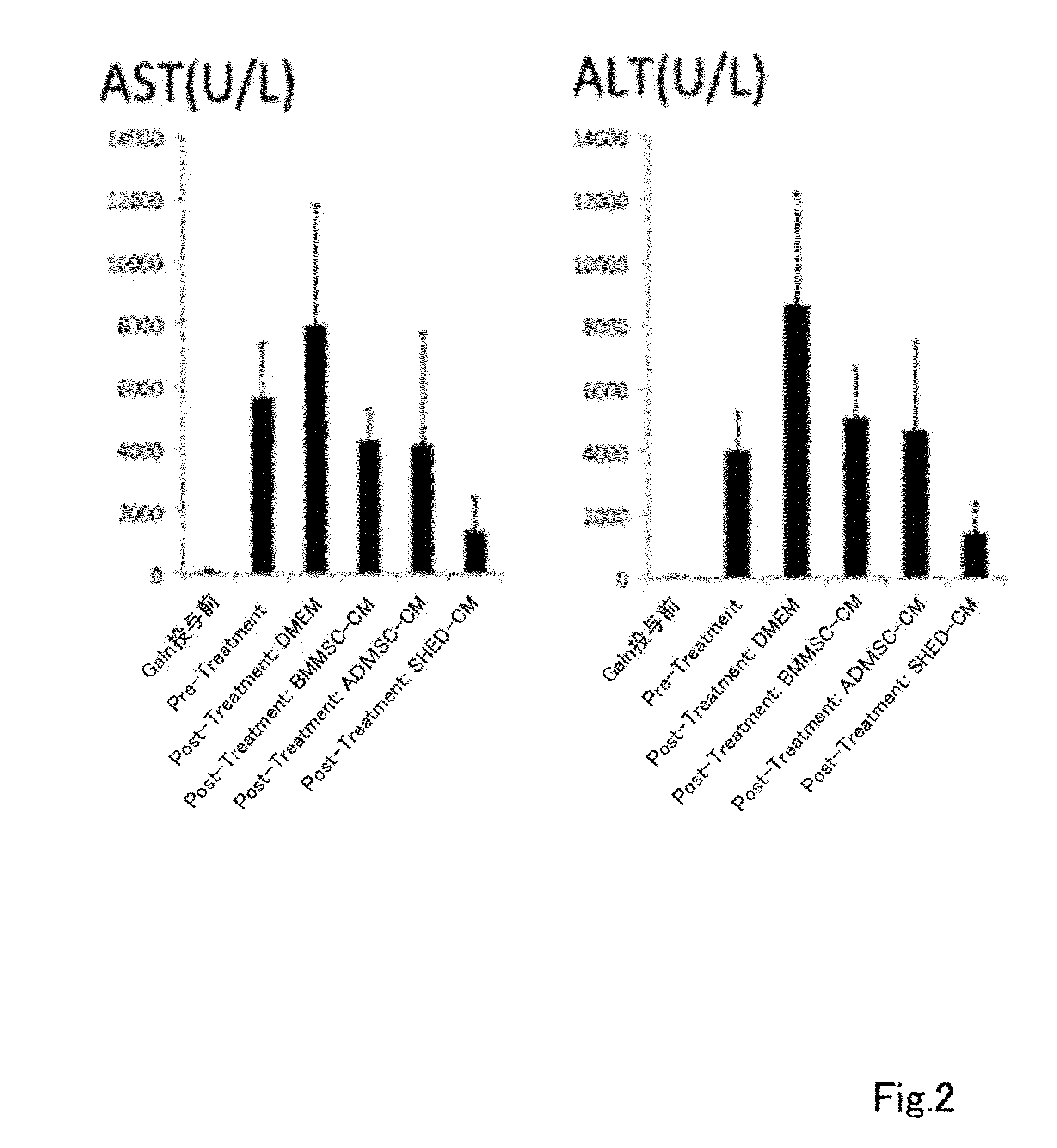
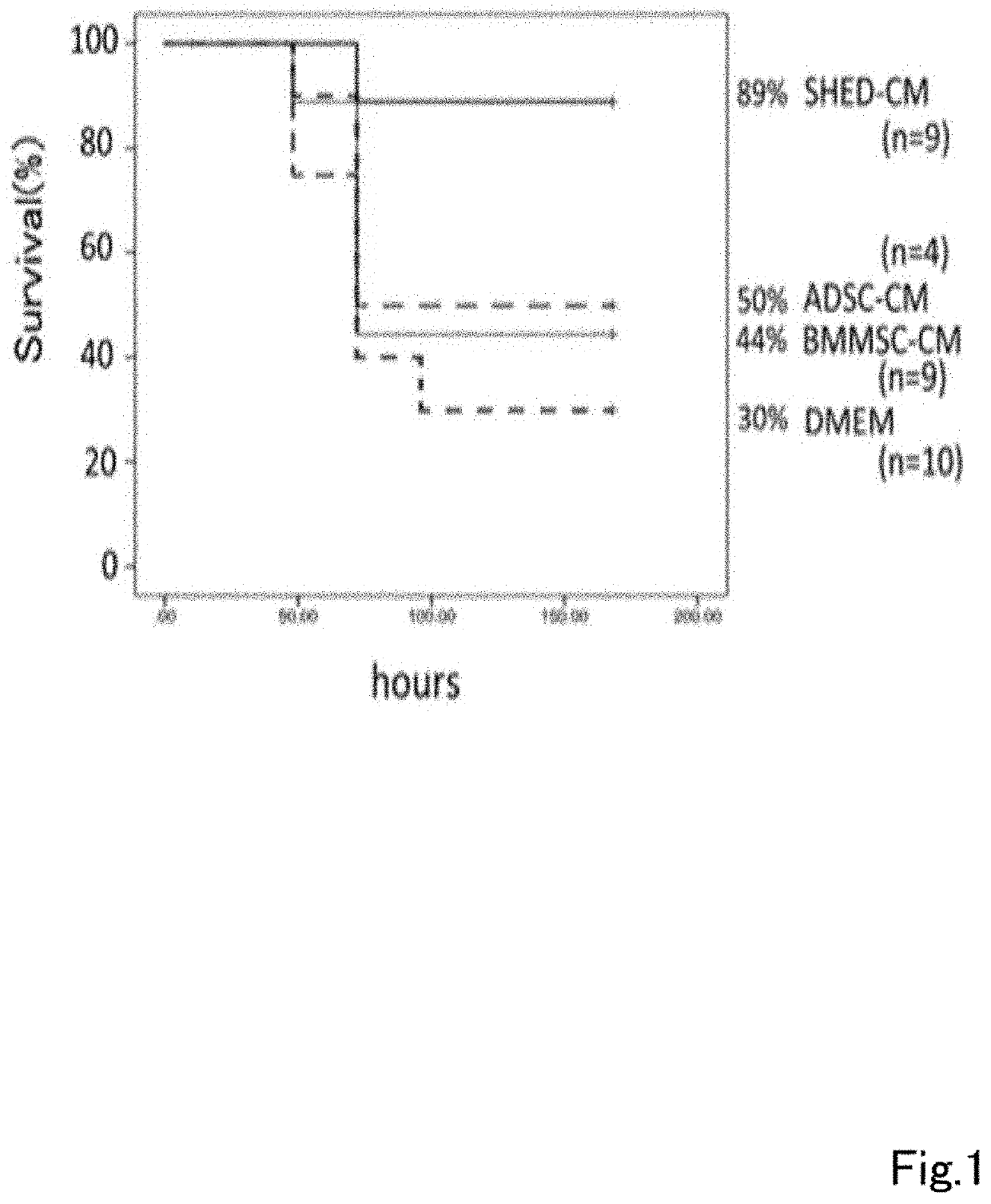
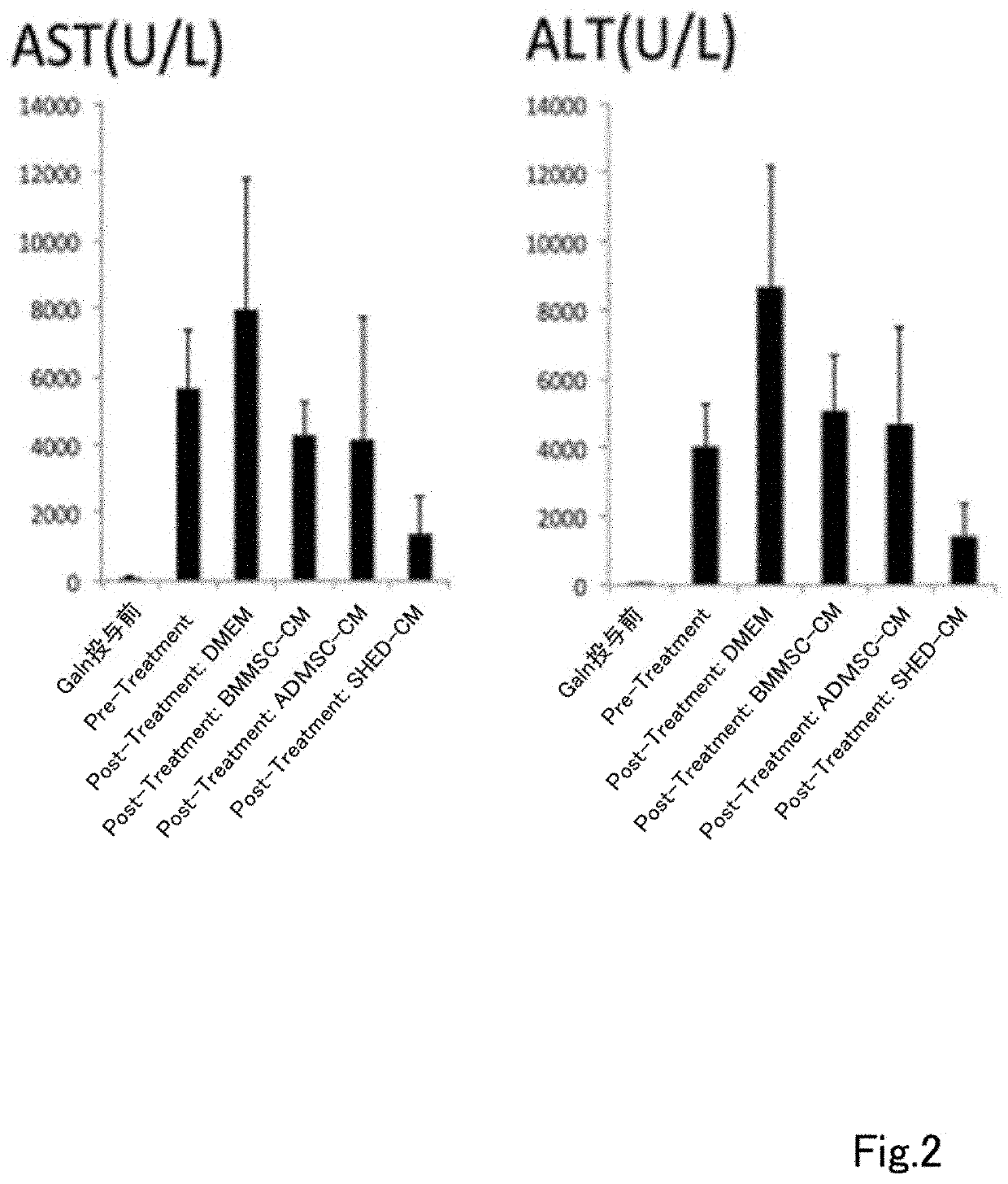
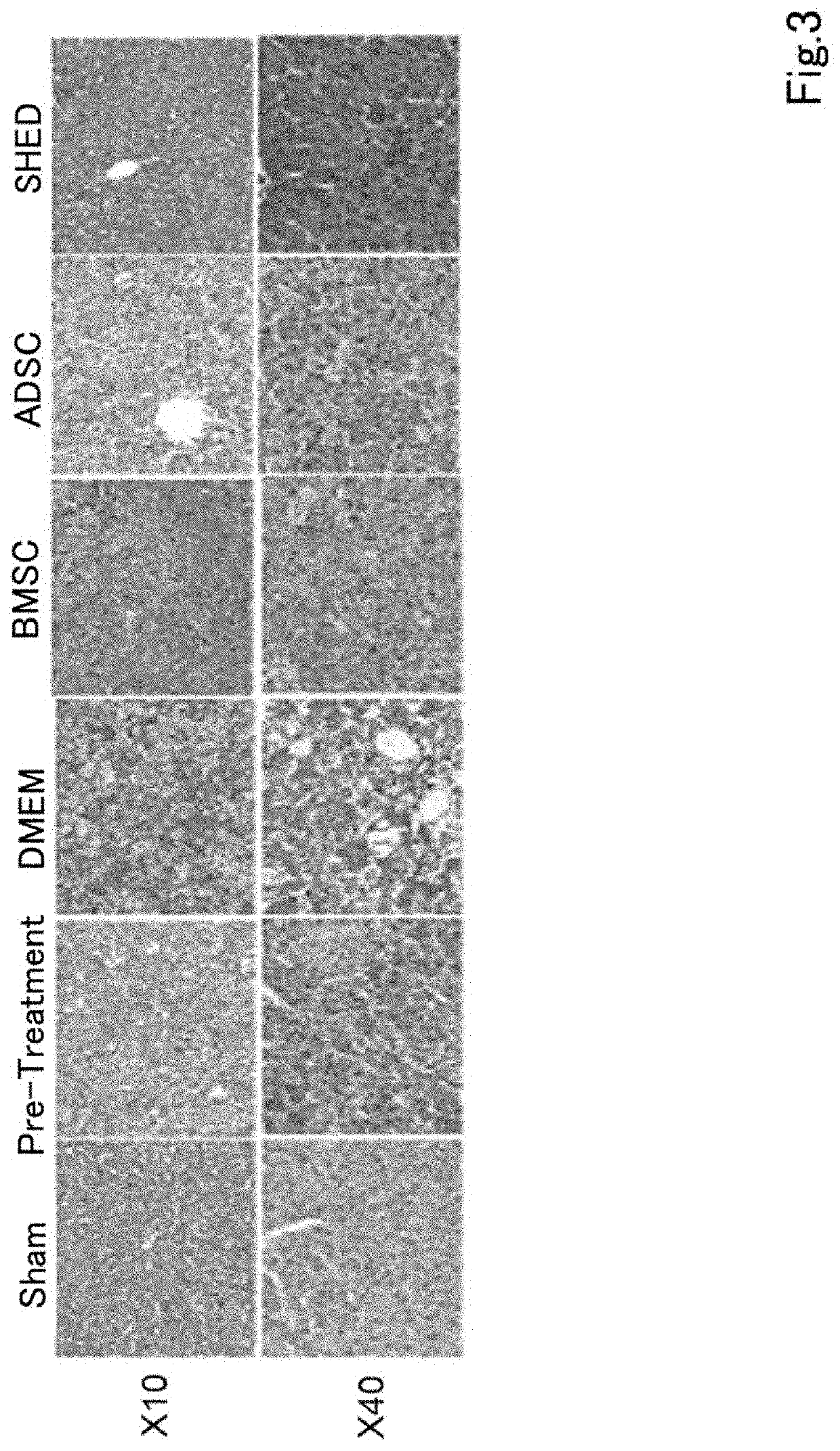
A composition for preventing or treating inflammatory disease and that is effective for inflammatory disease such as fulminant hepatitis and interstitial pneumonia. For such an objective, the present uses a culture supernatant obtained by culturing dental pulp stem cells as the active ingredient of the composition for preventing or treating inflammatory disease.
Owner:SHED TECH CORPORATION
Urchin yellow polysaccharide with liver protecting function and application thereof
InactiveCN104497162AHas hepatoprotective effectImprove survival rateDigestive systemImmunological disordersInflammatory factorsPerihepatitis
The invention discloses an urchin yellow polysaccharide with liver protecting function, and a preparation method and application thereof. The extraction separation technique of urchin yellow polysaccharide is improved: on the basis of the existing preparation method, a DEAE Sepharose Fast Flow filler with preferable performance is adopted, ultrafiltration is carried out before the DEAE Sepharose Fast Flow ion-exchange column chromatography to perform impurity removal, and meanwhile, ultrafiltration is adopted after Sephacryl-400 gel column chromatography instead of the existing dialysis. The invention lays emphasis on application of the urchin yellow polysaccharide in preparing drugs for preventing and / or treating fulminant hepatitis. The pharmacological experimental research indicates that the urchin yellow polysaccharide disclosed by the invention can obviously enhance the survival rate of mice with fulminant hepatitis, obviously lowers the contents of the serum AST and ALT and the hepatic tissue MDA of the mice with fulminant hepatitis, enhances the liver SOD, GSH-Px and CAT activity and GSH activity, obviously lowers the level of pro-inflammatory factors TNF-alpha, IL-1beta, IL-6 and NO, enhances the level of the anti-inflammatory factor IL-10, has obvious protective action on liver injury, and is hopeful to be developed into new drugs or health-care products with liver protecting function.
Owner:CHINA PHARM UNIV
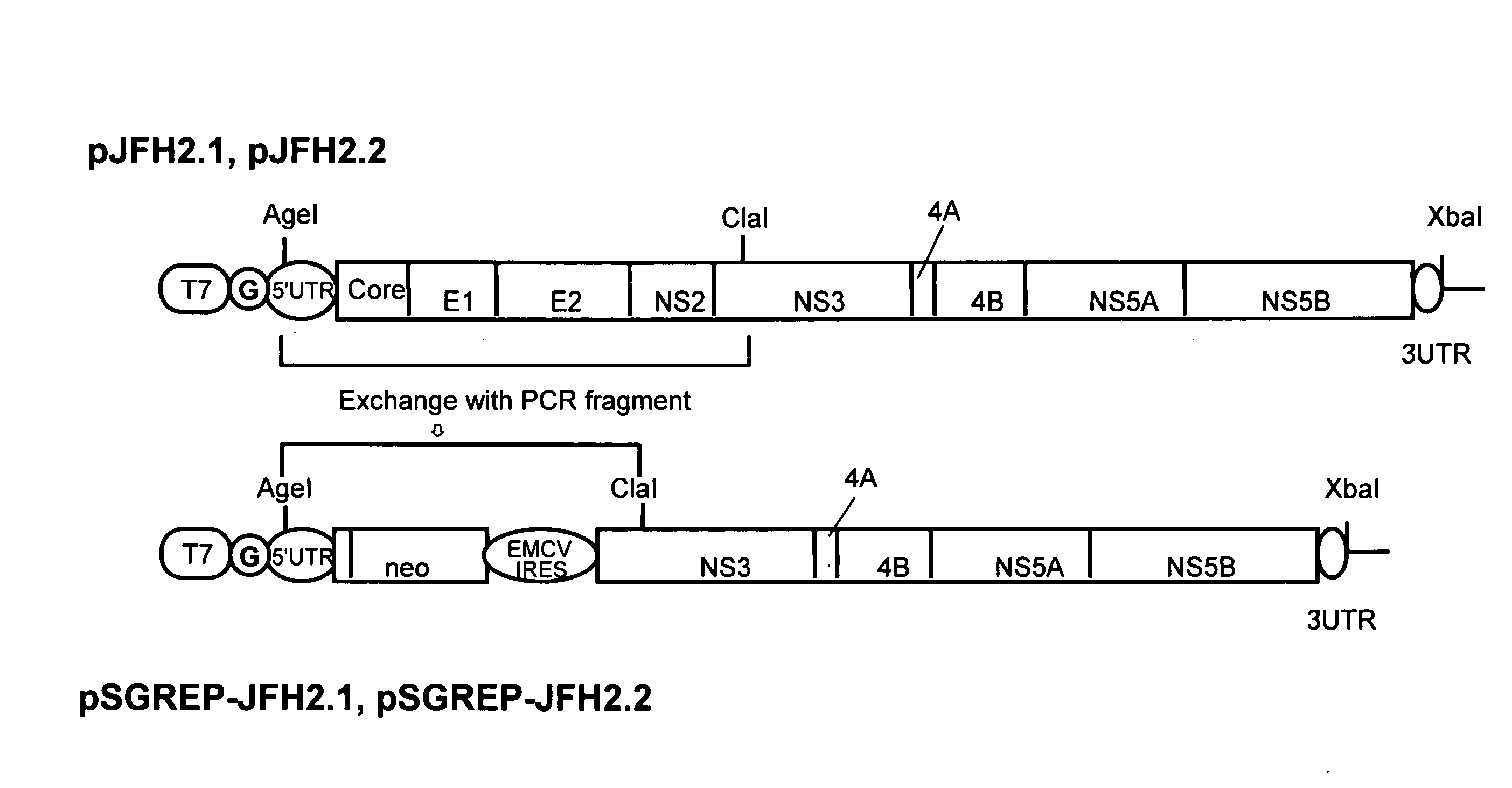
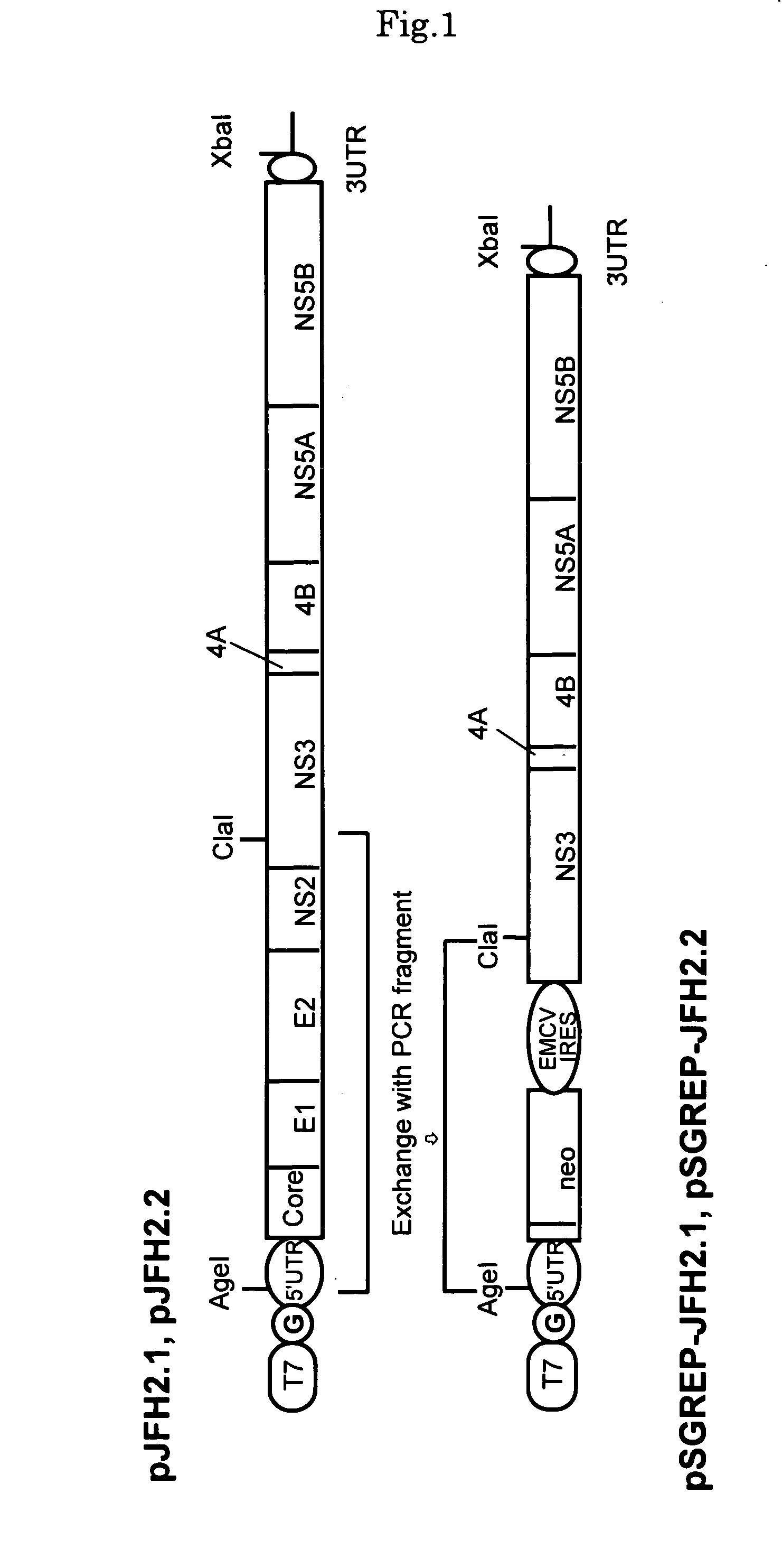
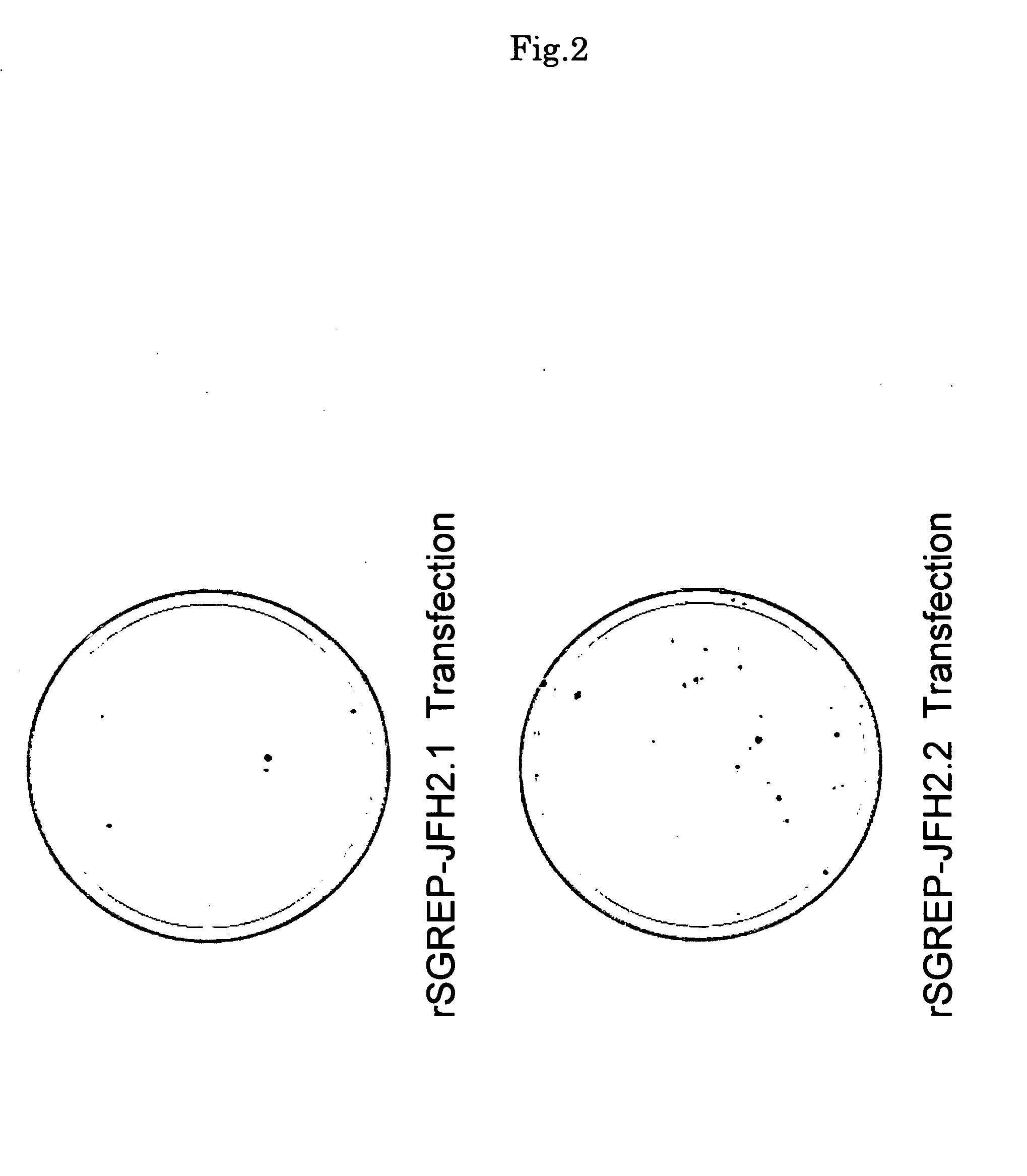
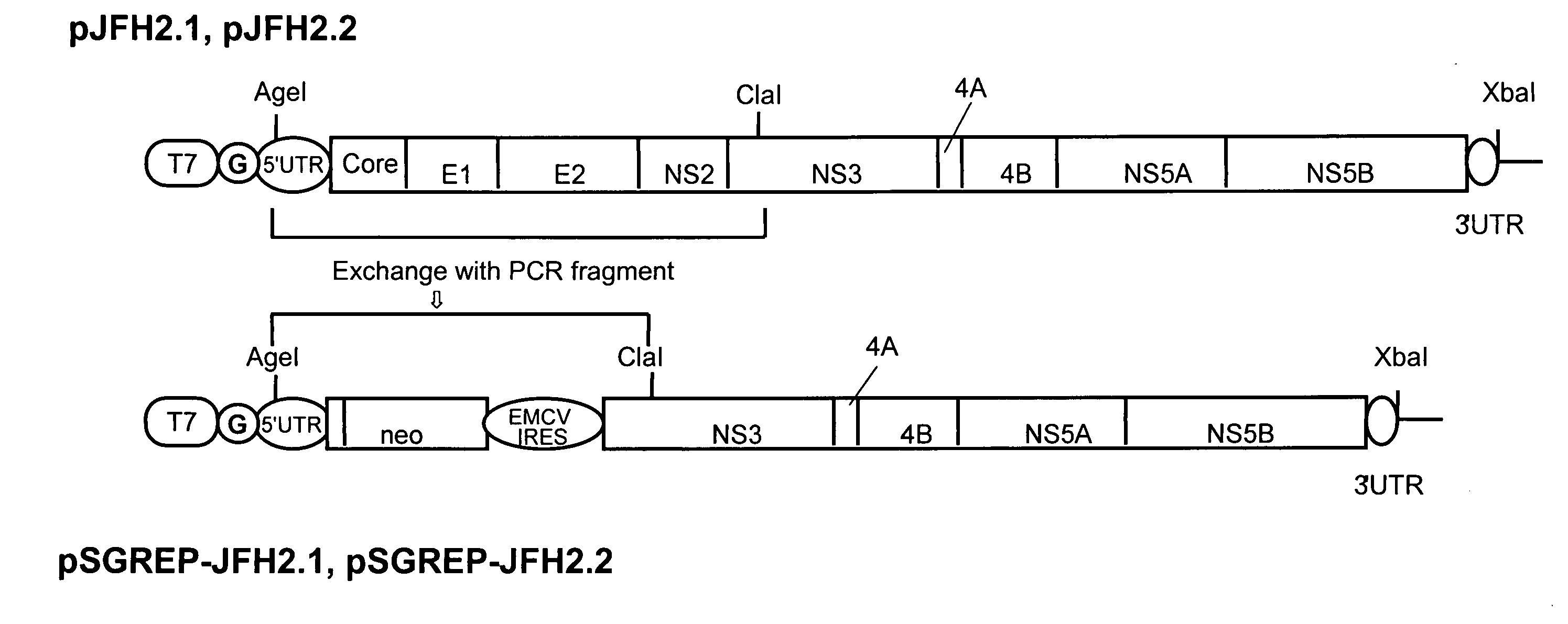
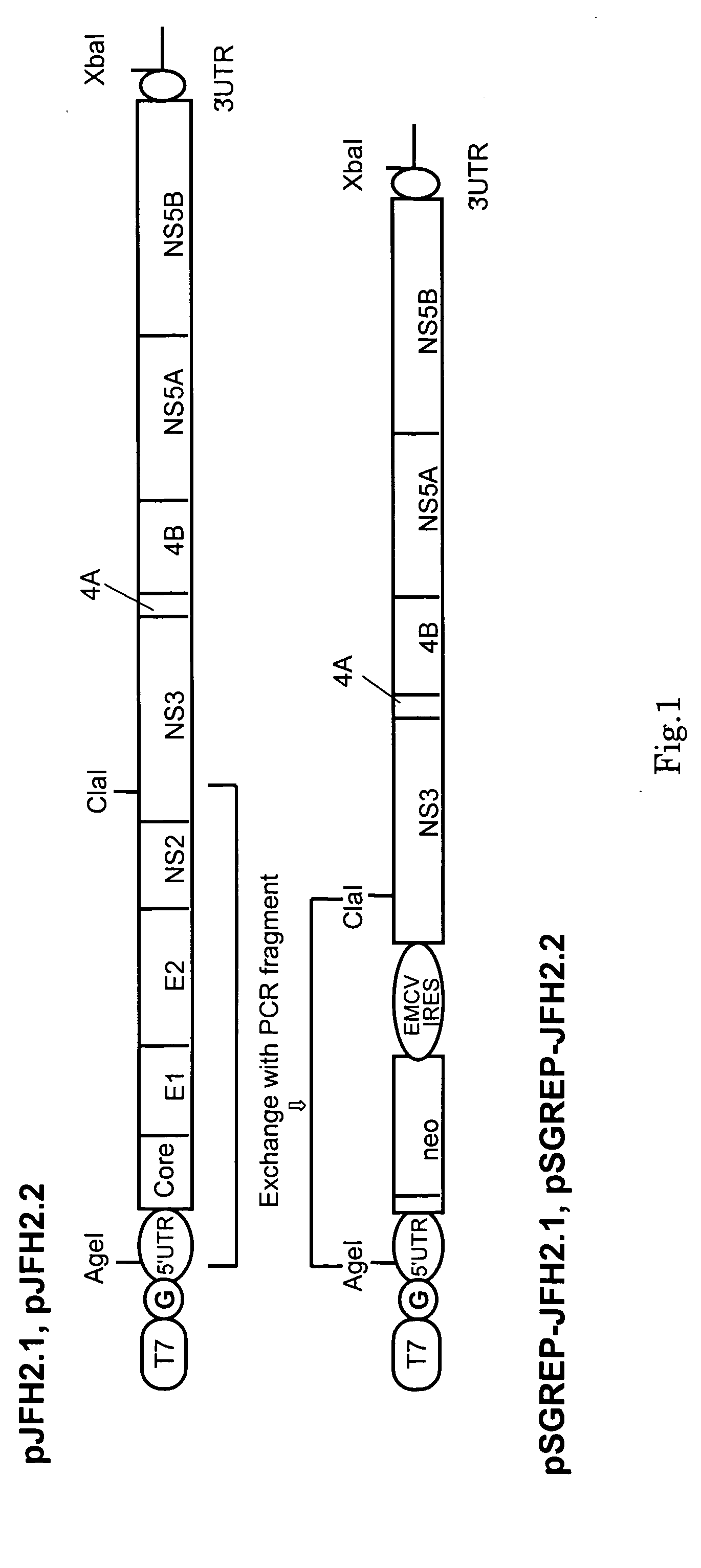
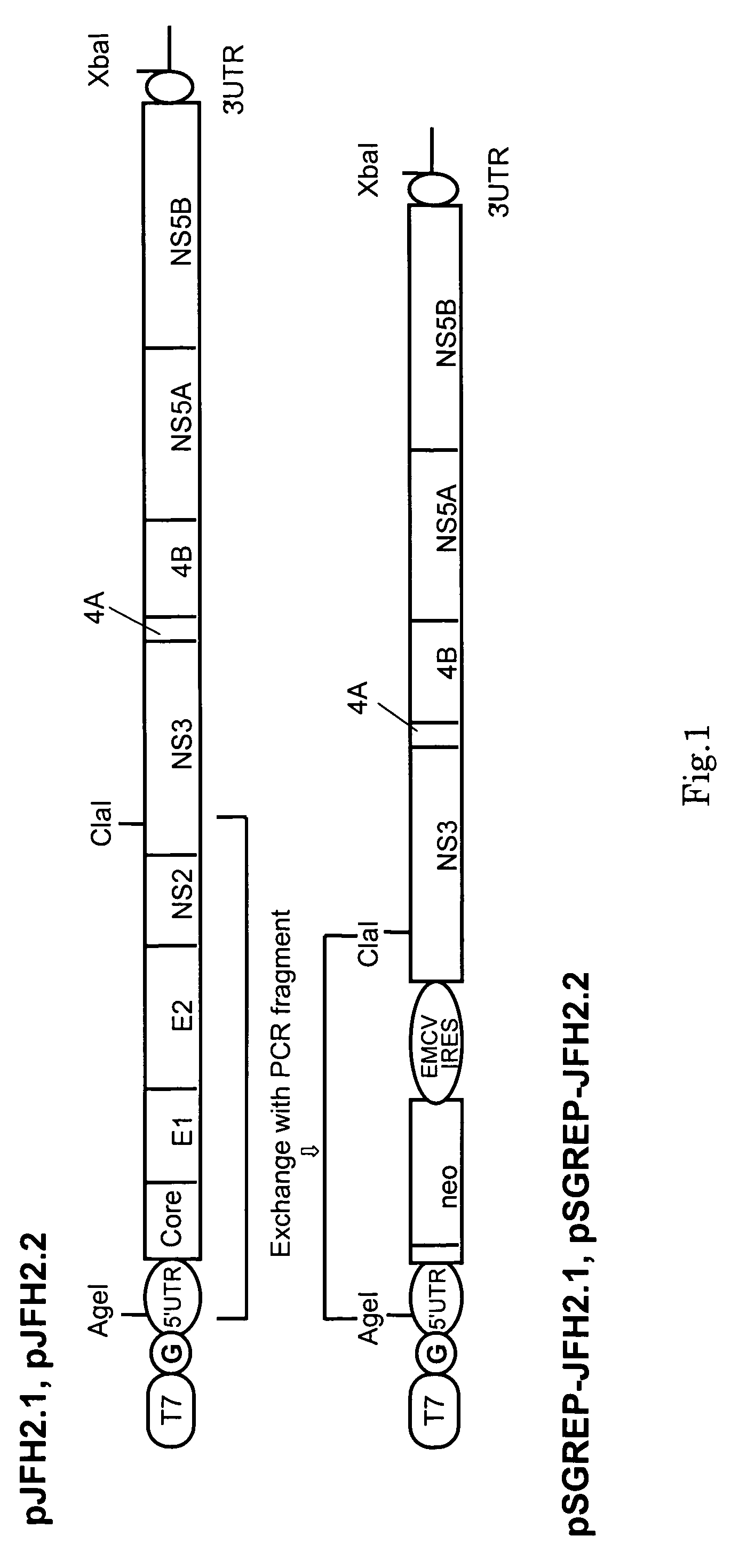
Nucleic Acid and Gene Derived from Novel HCV Strain and Replicon-Replicating Cell Using Said Gene
InactiveUS20090035747A1Improve efficiencyContinuous productionSsRNA viruses positive-sensePeptide/protein ingredientsVirus strainHcv replicon
The present invention relates to a gene derived from a novel fulminant hepatitis C virus strain, an HCV replicon RNA with a high replication efficiency obtained using the gene, and an HCV replicon-replicating cell transfected with the replicon RNA. When the HCV replicon RNA and the HCV replicon-replicating cell of the present invention are used, HCV proteins can be continuously produced in a large amount.
Owner:TOKYO METROPOLITAN INST OF MEDICAL SCI +1
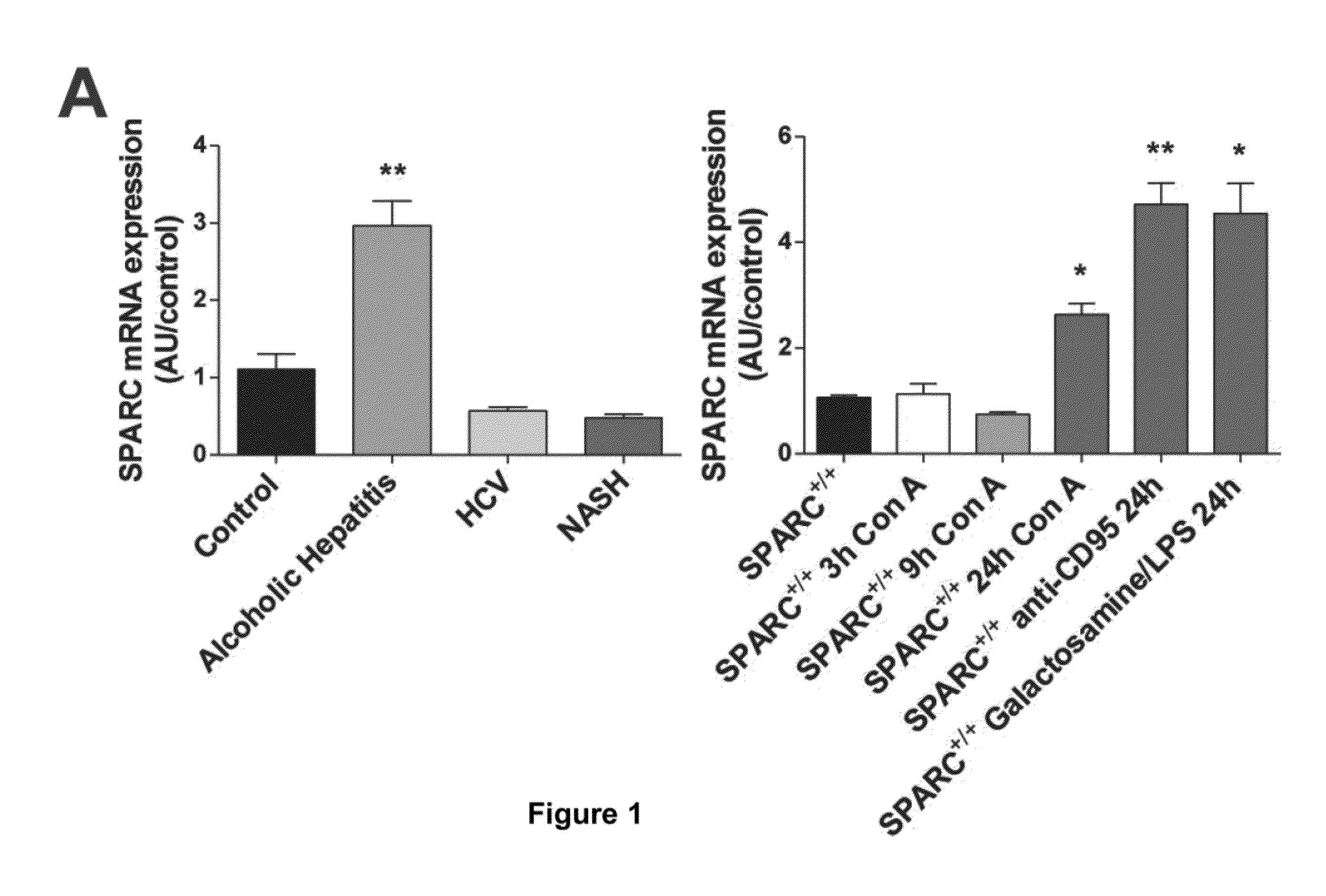
SPARC (Secreted Protein, Acidic and Rich in Cysteine), A New Target for the Treatment and Prevention of Acute Liver Failure
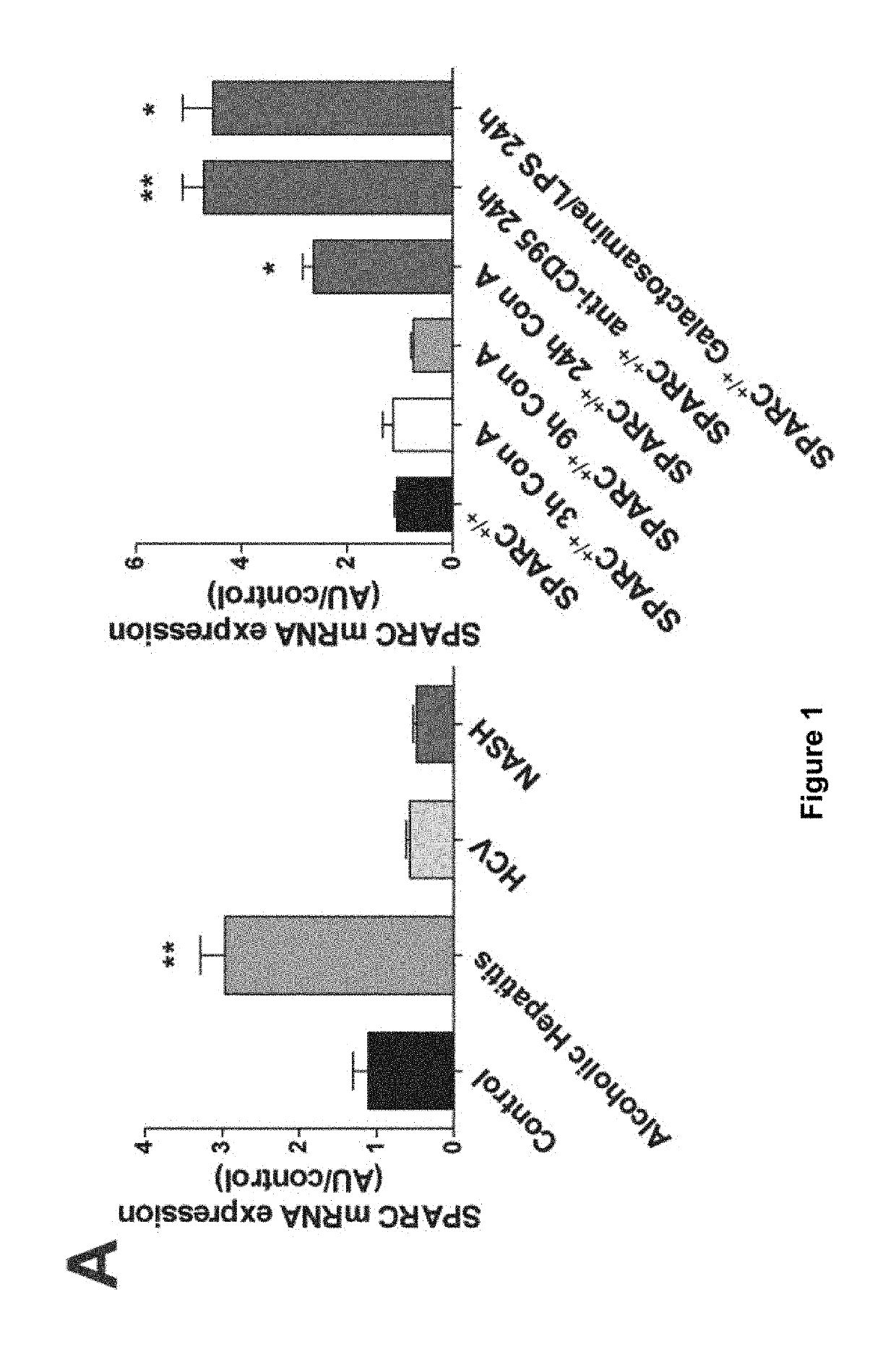
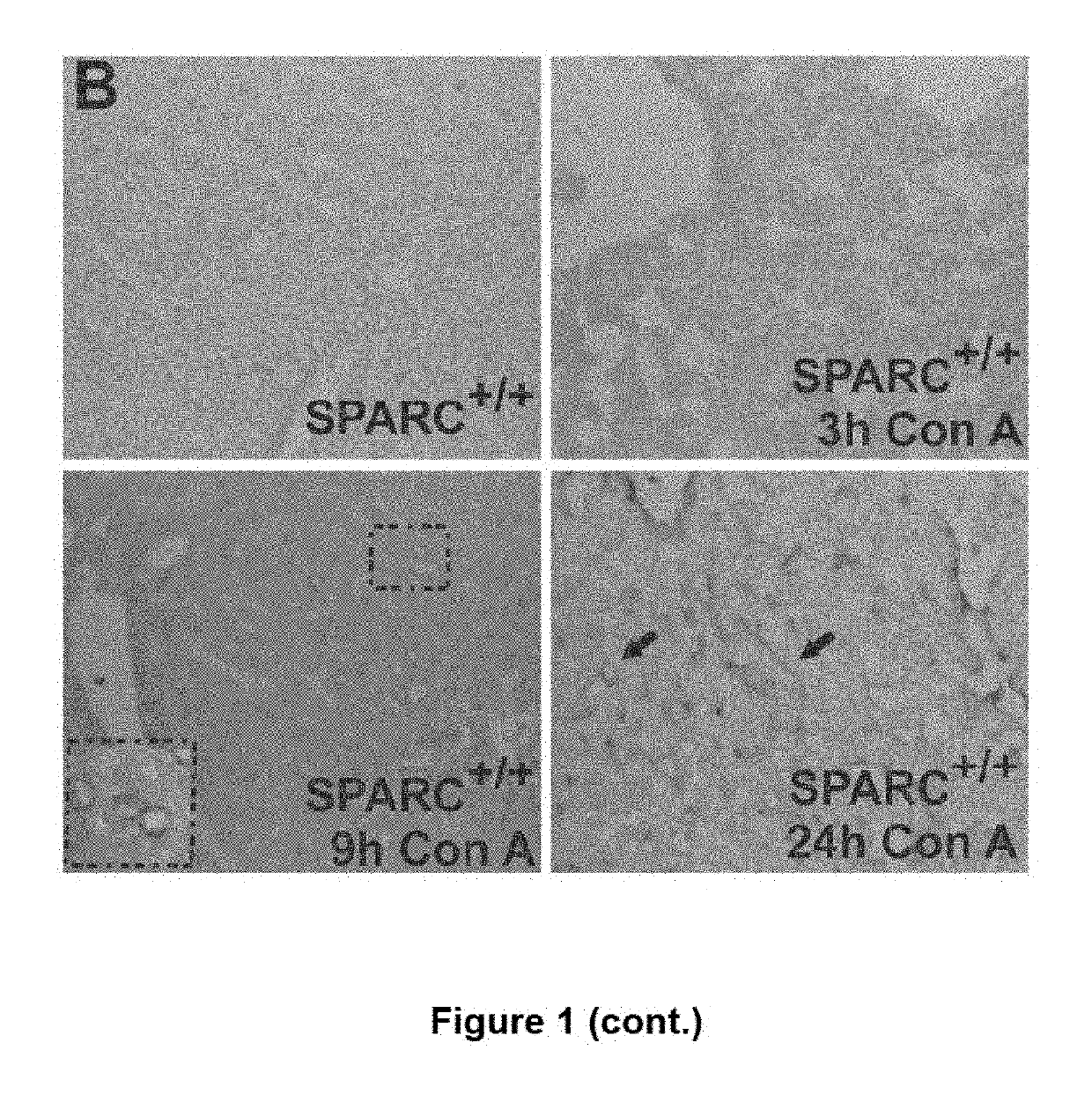
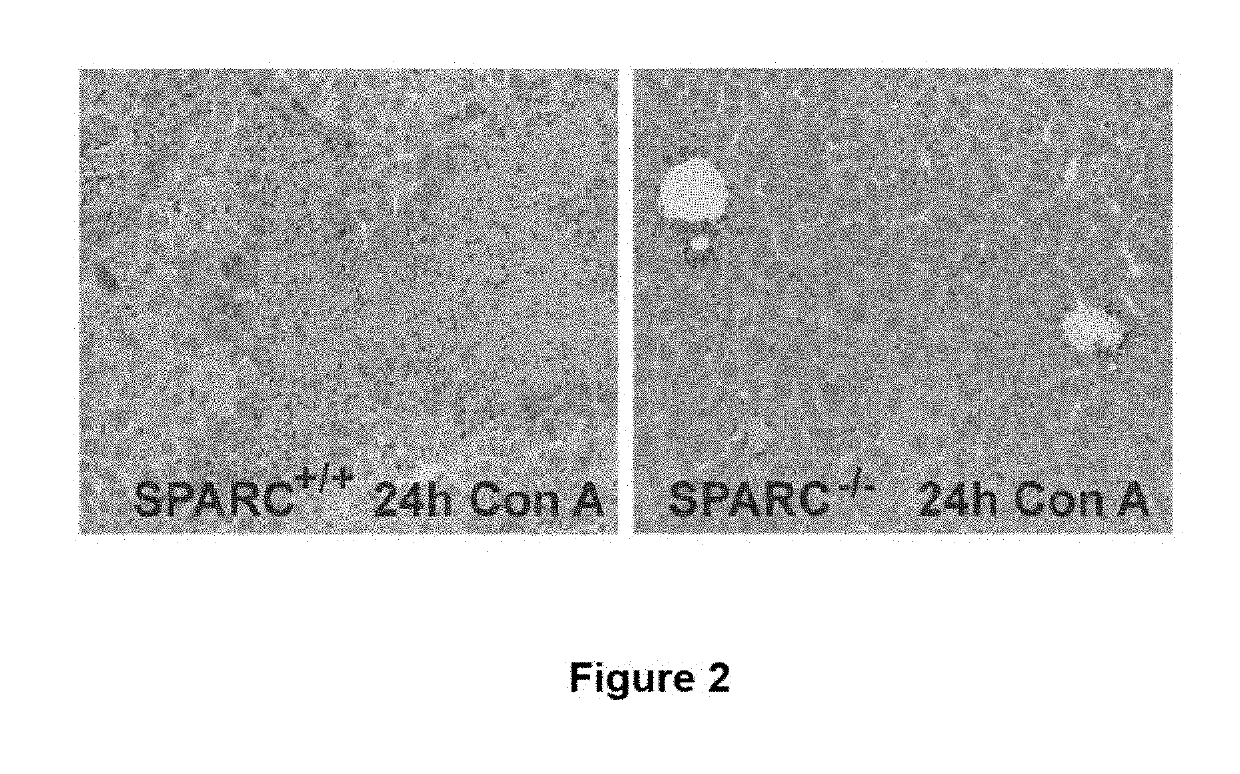
The invention relates to the identification of Secreted Protein, Acidic and Rich in Cysteine (SPARC) as a new therapeutic target in patients with fulminant hepatitis and allows the development of a strategy destined to protect the liver form damage. The invention relates to the treatment of acute liver failure or fulminant hepatitis by administering to a subject in need thereof an agent that inhibits at least partially the expression of SPARC and / or interferes with its biological function.
Owner:INIS BIOTEC LLC +3
Drug for preventing and controlling fulminant hepatitis
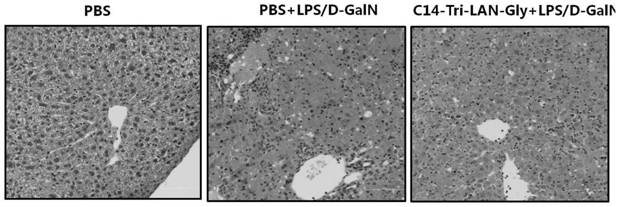
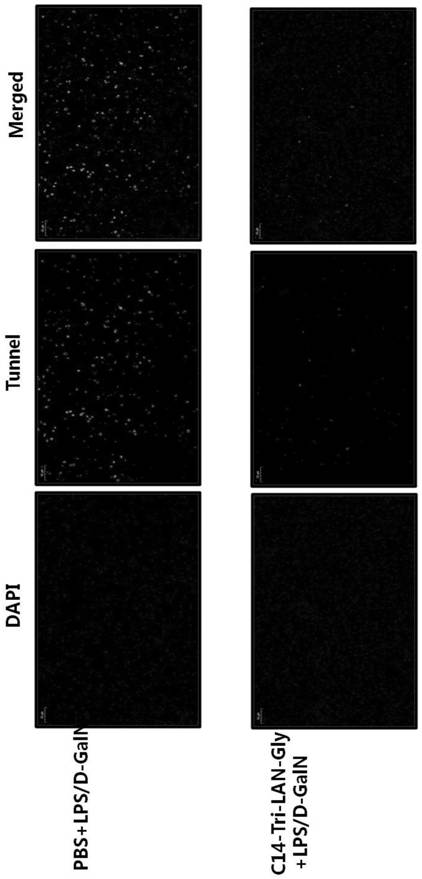
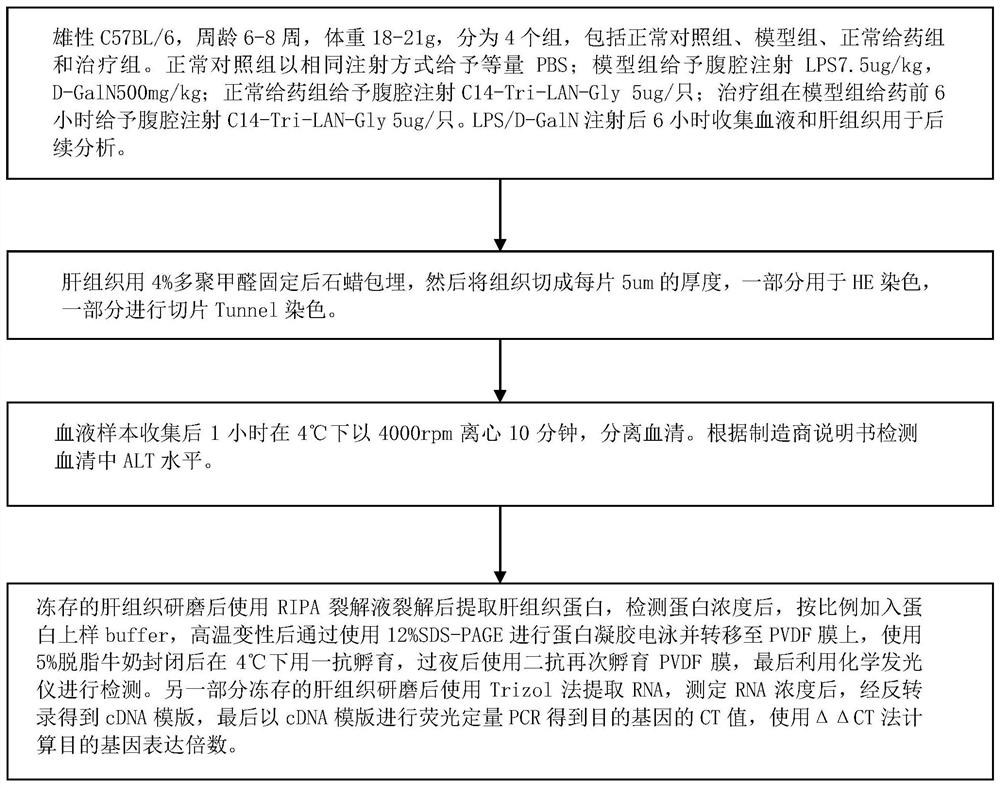
ActiveCN110694069ALower serum ALT levelsReduce apoptosisDigestive systemPharmaceutical active ingredientsLiver tissueLiver necrosis
The invention relates to the technical field of medicines, in particular to a drug for preventing and controlling fulminant hepatitis. Research shows the expression of the liver tissue A20 can be regulated up greatly by the NOD1 receptor agonist; the A20 (also called TNFAIP3) refers to anti-inflammatory signaling molecule, and can achieve anti-apoptosis action by lowering NF-kB signal channel, soas to protect the liver cells. Besides, the NOD1 receptor agonist can decrease the serum ALT level and the liver tissue cl-caspase3 level of mice with fulminant hepatitis greatly, thereby reducing liver injury of the mice with fulminant hepatitis and decreasing apoptosis of the liver cells. The conclusion is the NOD1 receptor agonist can be applied to treating fulminant hepatitis.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
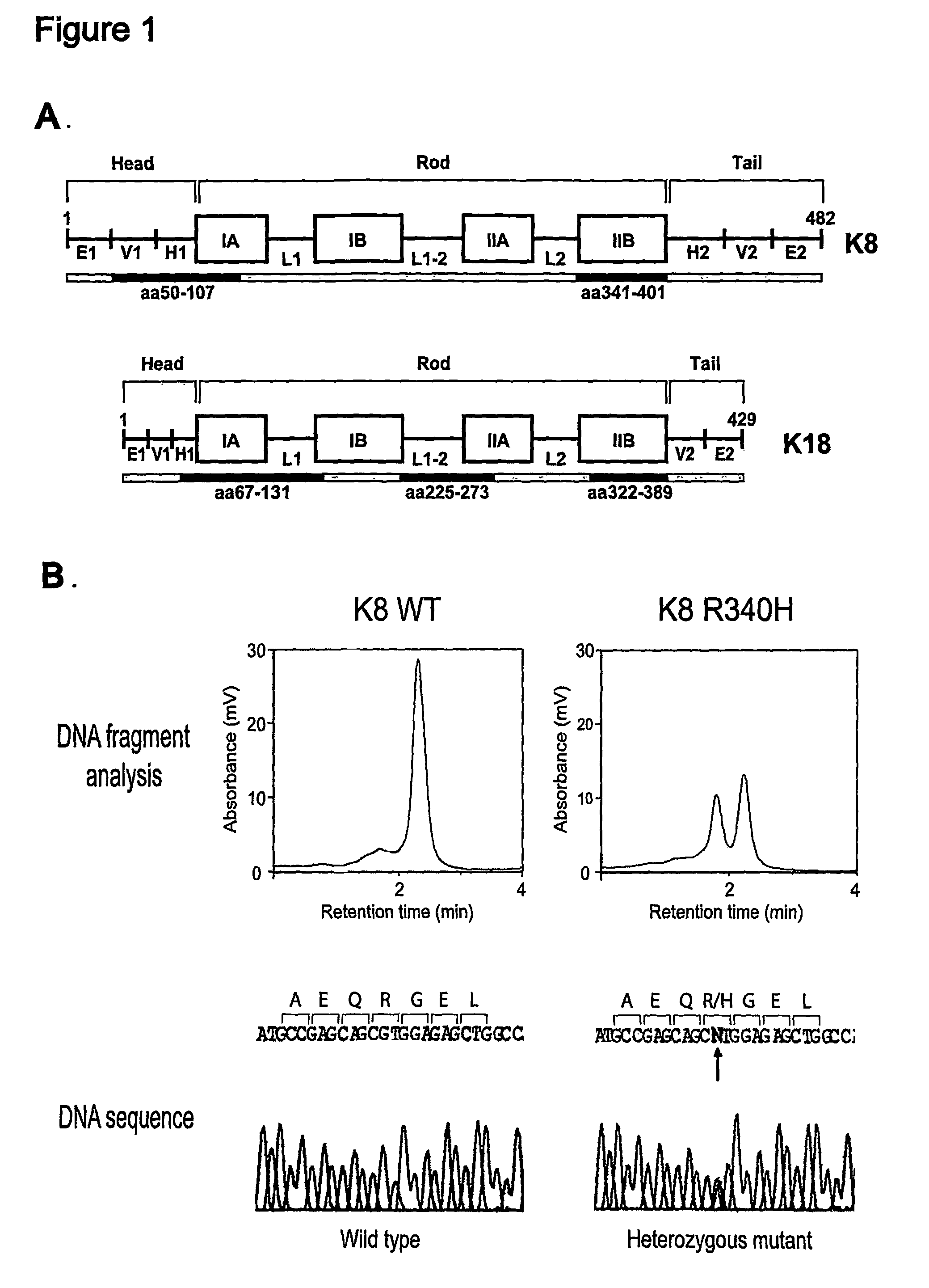
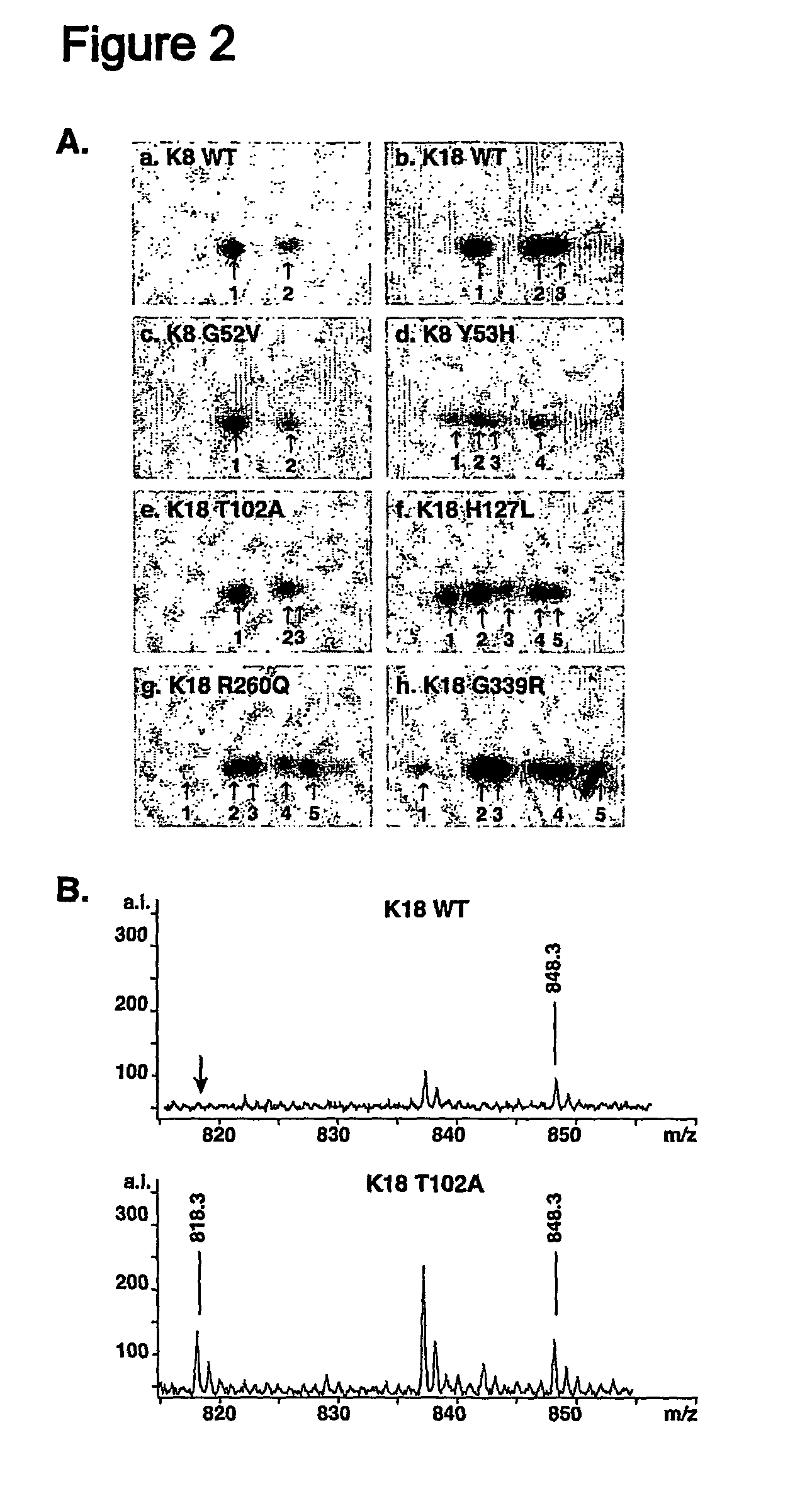
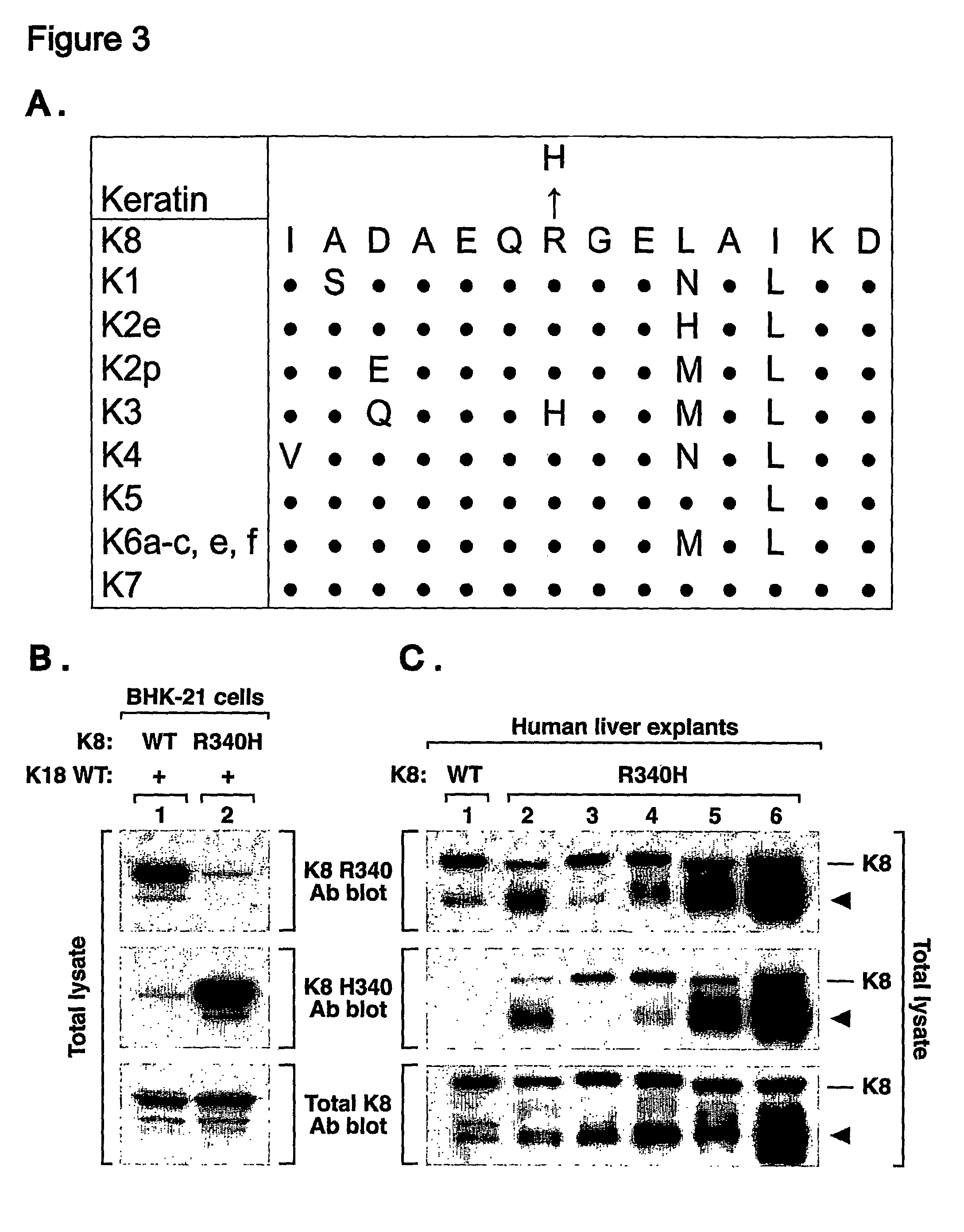
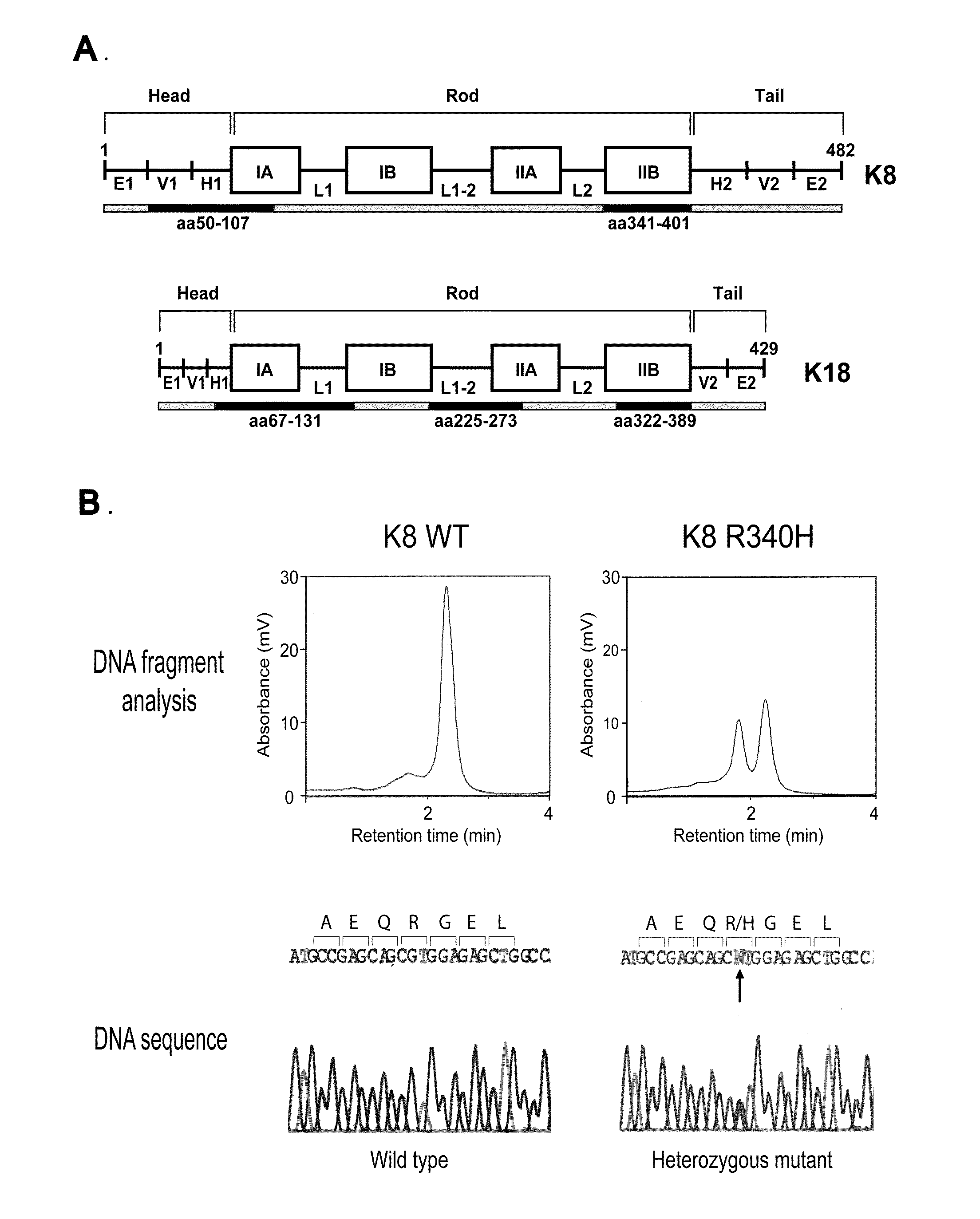
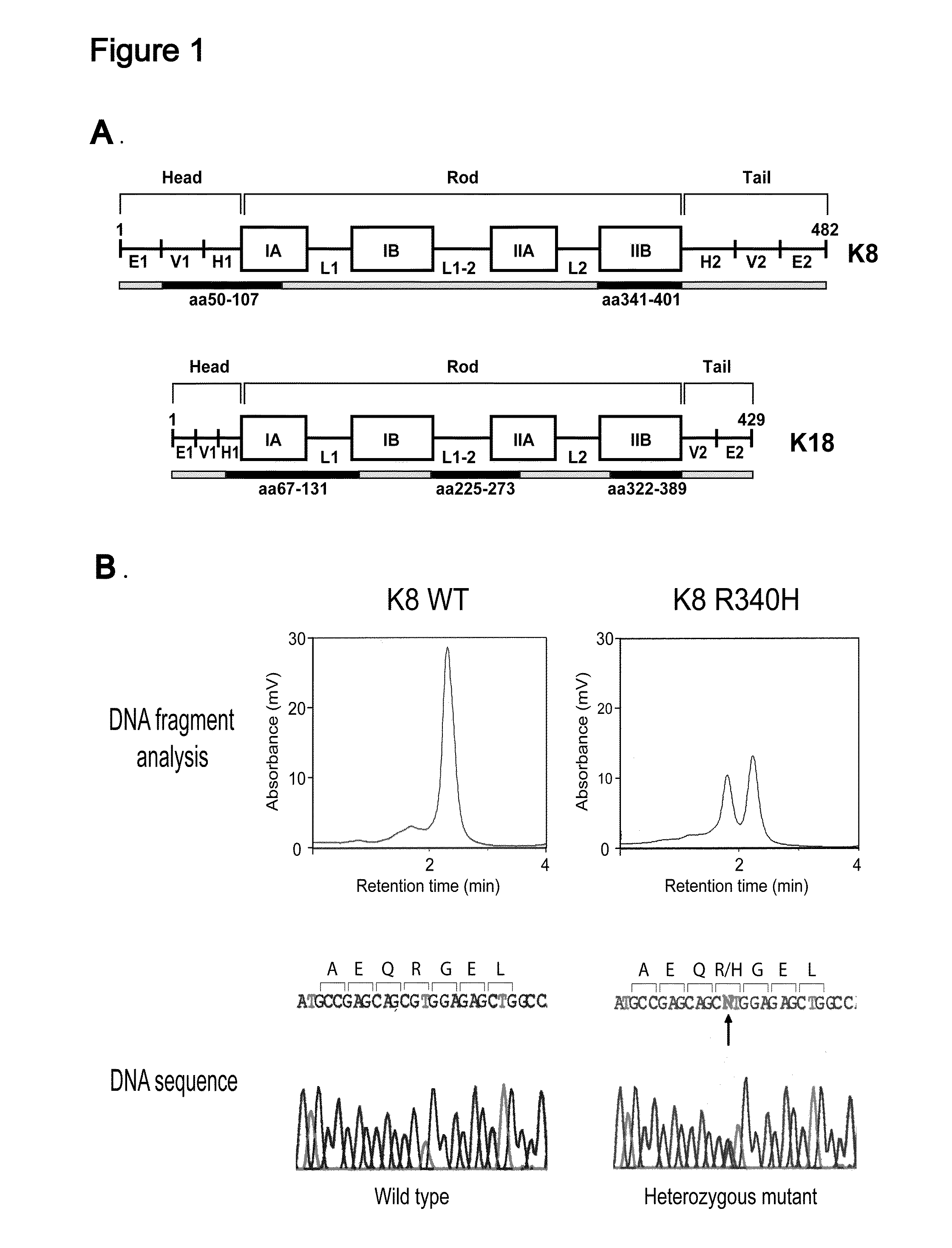
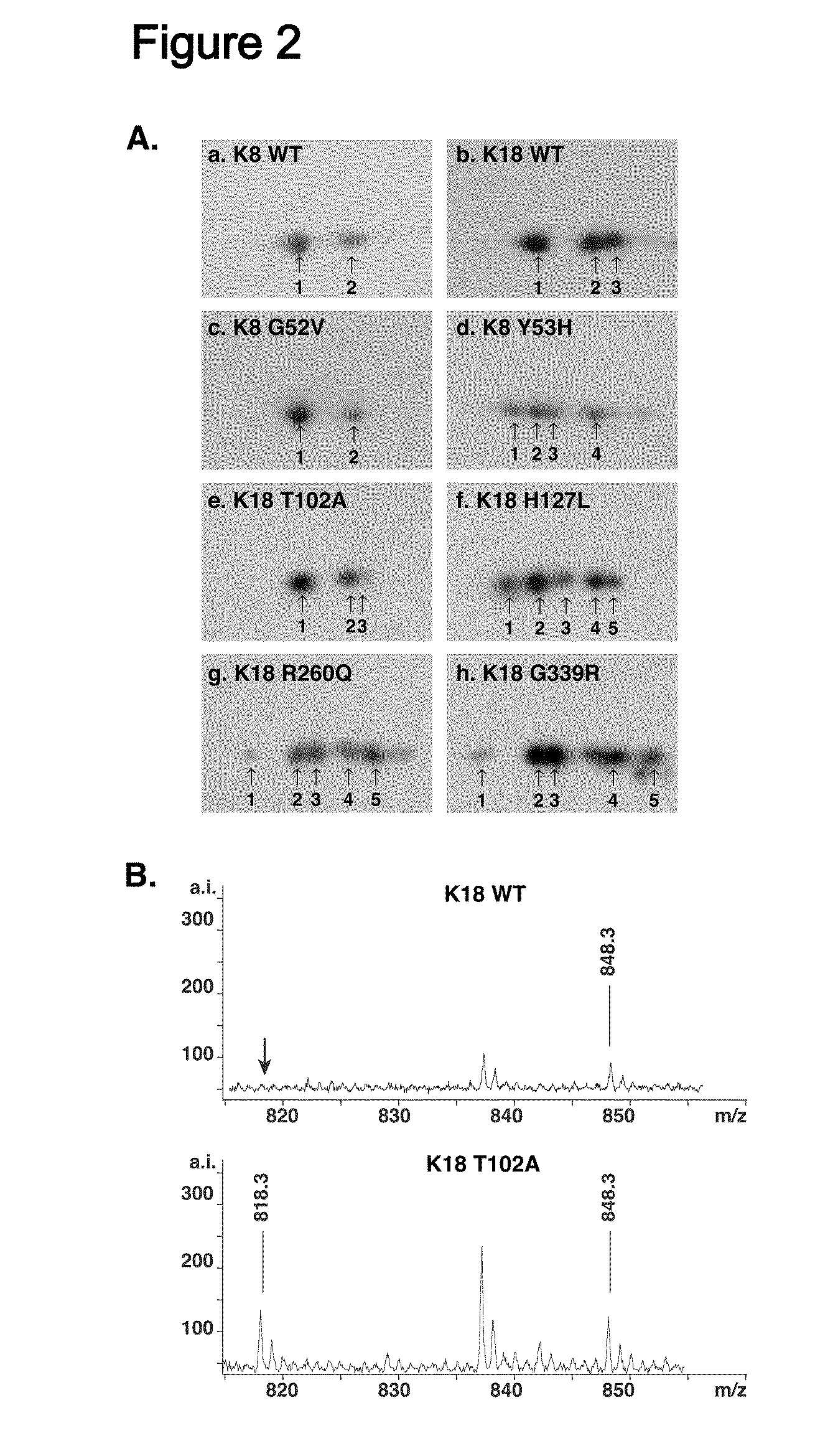
Keratin 8 mutations are risk factors for developing liver disease of multiple etiologies
ActiveUS7838217B1High incidenceModulate expressionSugar derivativesPeptide/protein ingredientsEtiologyHepatobiliary disease
Keratin 8 and 18 (K8 / K18) mutations are shown to be associated with a predisposition to liver or biliary tract disease, particularly noncryptogenic hepatobiliary disease. Unique K8 / K18 mutations are shown in patients with diseases including but without limitation to viral hepatitis, biliary atresia, alcoholic cirrhosis and other acute or chronic toxic liver injury, cryptogenic cirrhosis, acute fulminant hepatitis, autoimmune liver disease, cystic fibrosis, primary biliary cirrhosis, primary sclerosing cholangitis, diseases that are linked with cryptogenic cirrhosis, such as nonalcoholic steatohepatitis, and the like. Livers with keratin mutations had increased incidence of cytoplasmic filamentous deposits. Therefore, K8 / K18 are susceptibility genes for developing cryptogenic and noncryptogenic forms of liver disease. Mutant alleles are associated with disease susceptibility, and their detection is used in the diagnosis of a predisposition to these conditions.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
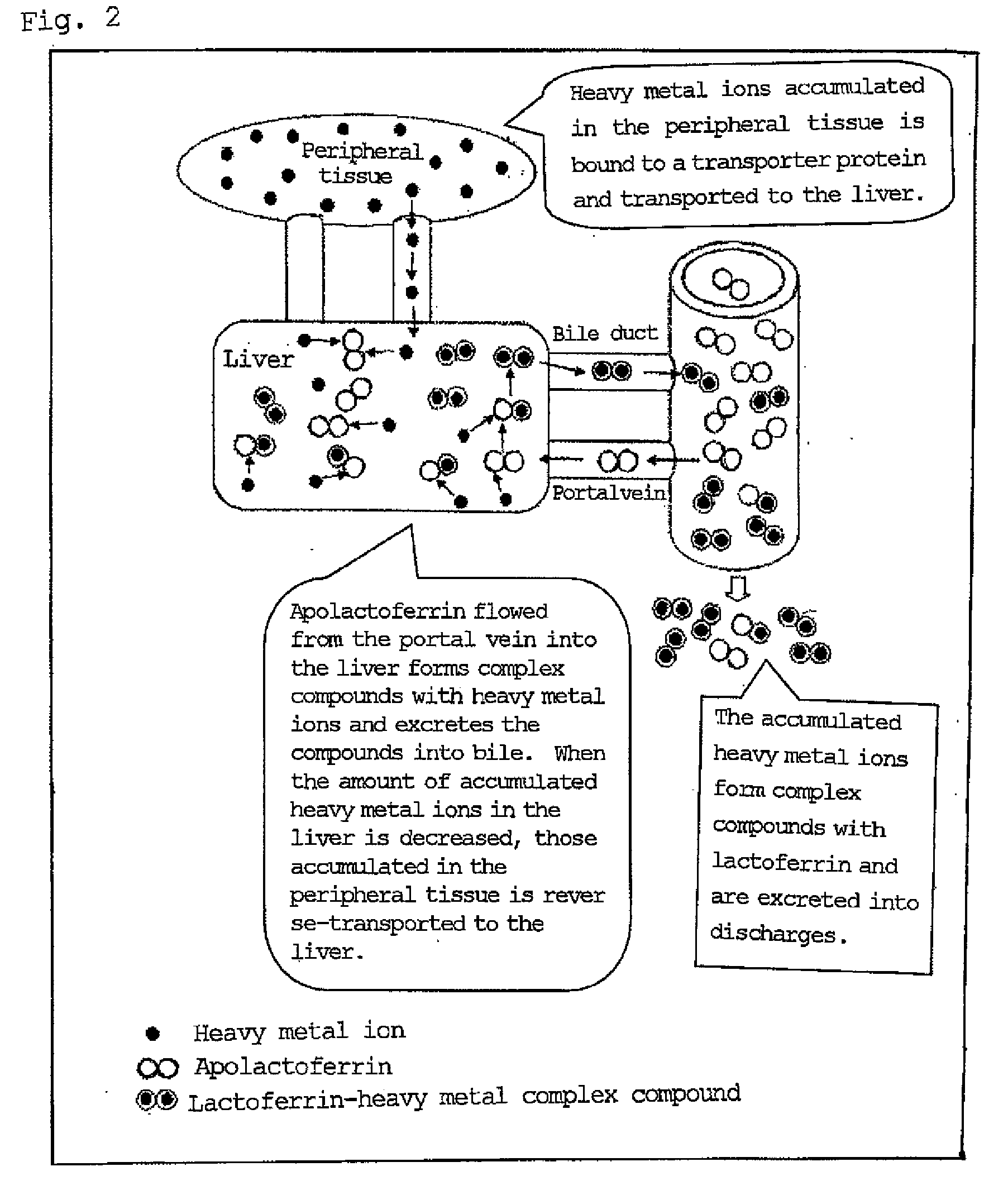
Agent for ameliorating heavy metal-induced disorders, and medicinal composition, food and cosmetic containing the same
InactiveUS20090082269A1Prevent and decrease accumulationEliminate and decrease its effectPeptide/protein ingredientsTransferrinsDiseaseSide effect
It is intended to provide a drug for ameliorating symptoms or diseases caused by heavy metals (for example, Wilson's disease, heavy metal toxication, aging, fulminant hepatitis and so on) which has a high safety without any fear of side effect, can eliminate heavy metals such as copper ion accumulated in excess in the living body to prevent or lessen the accumulation of the heavy metals in the living body, thereby eliminating or relieving the effects of the heavy metals; and compositions such as a medicinal composition, a food and a cosmetic containing the same. The drug and compositions as described above are characterized by containing lactoferrin and / or an active derivative of the same as the active ingredient.
Owner:NRL PHARMA
Method for building virus hepatitis mice model
The present invention is the method of establishing mouse model of viral hepatitis. The method includes selection of mouse hepatitis virus-III (MHV-3) of 100-1000 pFu, injecting infection of variousvariety mice of BALB / CJ, C3H / HEJ and A / J mice, and observing the survival rate and liver tissue change. Via the said process, three kinds of viral hepatitis mouse model are established, of fulminanthepatitis, chronic hepatitis and symptom-free mouse separately. The establishment of the model provides powerful facilities for systematic research of viral hepatitis in genetic regulation, molecular mechanism and prevention and treatment.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Chinese medicine preparation for treating hepatitis and anti-fulminant hepatitis, and its preparing method and use
InactiveCN1977921BReduce pathological damageImprove rheologyDigestive systemAntiviralsMedicineChronic hepatitis
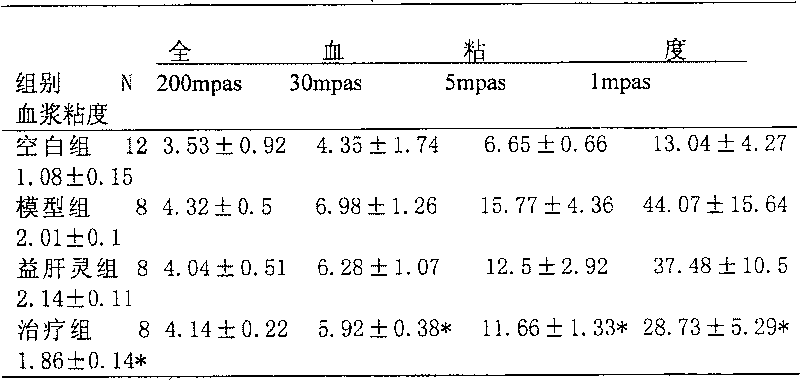
The present invention discloses a Chinese medicine preparation for curing hepatitis and resisting hepatic failure. Said Chinese medicine preparation is made up by using the Chinese medicinal materialsof red peony root, dried / fresh rehmannia root and rhubarb through a certain preparation process. Said invention also provides the concrete steps of said preparation process.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Nucleic acid and gene derived from novel HCV strain and replicon-replicating cell using said gene
InactiveUS8022197B2Improve efficiencyContinuous productionSsRNA viruses positive-senseVectorsVirus strainHcv replicon
The present invention relates to a gene derived from a novel fulminant hepatitis C virus strain, an HCV replicon RNA with a high replication efficiency obtained using the gene, and an HCV replicon-replicating cell transfected with the replicon RNA. When the HCV replicon RNA and the HCV replicon-replicating cell of the present invention are used, HCV proteins can be continuously produced in a large amount.
Owner:TOKYO METROPOLITAN INST OF MEDICAL SCI +1
Keratin 8 Mutations are Risk Factors for Developing Liver Disease of Multiple Etiologies
Keratin 8 and 18 (K8 / K18) mutations are shown to be associated with a predisposition to liver or biliary tract disease, particularly noncryptogenic hepatobiliary disease. Unique K8 / K18 mutations are shown in patients with diseases including but without limitation to viral hepatitis, biliary atresia, alcoholic cirrhosis and other acute or chronic toxic liver injury, cryptogenic cirrhosis, acute fulminant hepatitis, autoimmune liver disease, cystic fibrosis, primary biliary cirrhosis, primary sclerosing cholangitis, diseases that are linked with cryptogenic cirrhosis, such as nonalcoholic steatohepatitis, and the like. Livers with keratin mutations had increased incidence of cytoplasmic filamentous deposits. Therefore, K8 / K18 are susceptibility genes for developing cryptogenic and noncryptogenic forms of liver disease. Mutant alleles are associated with disease susceptibility, and their detection is used in the diagnosis of a predisposition to these conditions.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
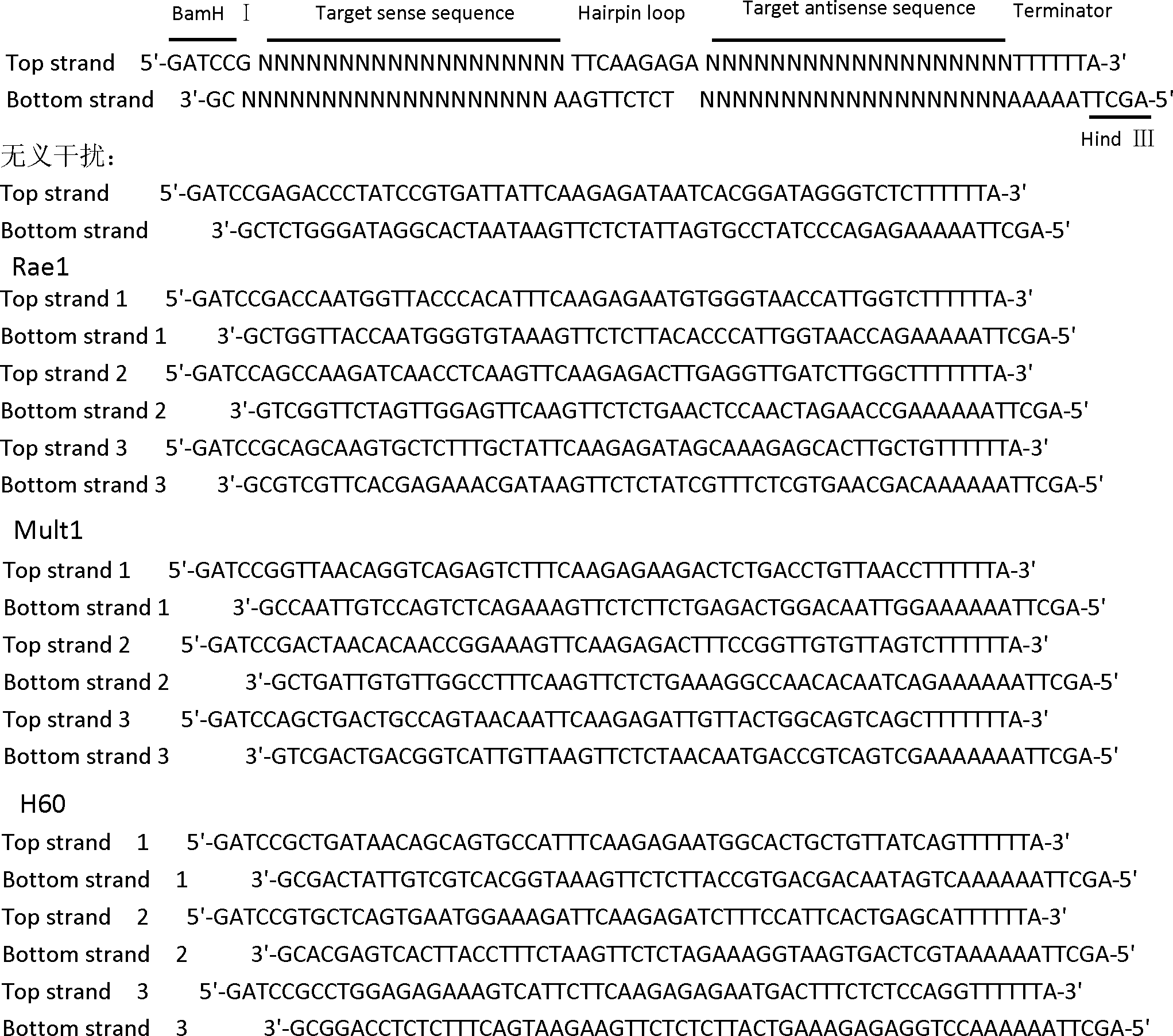
RNA interference vector capable of simultaneously silencing multiple ligands of NKG2D, and construction method and application thereof
ActiveCN102505022ADownregulate the local environmentImprove protectionGenetic material ingredientsDigestive systemMulti targetingBiology
The invention discloses a method for constructing a RNA interference vector capable of simultaneously silencing multiple ligands of NKG2D, and application of the RNA interference vector. The method constructs a single vector capable of encoding shRNAs of multiple ligand molecules (Rae1, Mult1 and H60) of NKG2D. The vector can be further constructed to produce an adenovirus vector encoding shRNAs of multiple ligands of NKG2D and having an infectious ability. The vector can silence different target genes at the same time, to achieve the purpose of disease treatment. The vector can be applied to lower liver local microenvironment, including lowering the expression levels of Rae1, Mult1 and H60 on the surfaces of liver parenchymal cells, and is significant in prevention and treatment of NKG2D-dependent fulminant hepatitis. The invention develops the multi-target RNA interference technique and has important practical significance in multi-target gene therapy.
Owner:UNIV OF SCI & TECH OF CHINA
Nucleic acid and gene derived from novel HCV strain and replicon-replicating cell using said gene
InactiveUS20090042181A1Improve replication efficiencyIncrease probabilitySsRNA viruses positive-sensePeptide/protein ingredientsRNA transfectionVirus strain
The present invention relates to a gene derived from a novel fulminant hepatitis C virus strain, an HCV replicon RNA with a high replication efficiency obtained using the gene, and an HCV replicon-replicating cell transfected with the replicon RNA. When the HCV replicon RNA and the HCV replicon-replicating cell of the present invention are used, HCV proteins can be continuously produced in a large amount.
Owner:TORAY IND INC +1
Nucleic acid and gene derived from novel HCV strain and replicon-replicating cell using said gene
InactiveUS7790448B2Improve efficiencyContinuous productionSsRNA viruses positive-senseSugar derivativesRNA transfectionVirus strain
The present invention relates to a gene derived from a novel fulminant hepatitis C virus strain, an HCV replicon RNA with a high replication efficiency obtained using the gene, and an HCV replicon-replicating cell transfected with the replicon RNA. When the HCV replicon RNA and the HCV replicon-replicating cell of the present invention are used, HCV proteins can be continuously produced in a large amount.
Owner:TORAY IND INC +1
Galactolipids-enriched plant extracts and the uses thereof
ActiveUS20190060388A1Promoting skin whiteningAntibacterial agentsCosmetic preparationsAdditive ingredientMedicine
The present invention is related to a galactolipids-enriched plant extract, prepared by extracting a plant sample selected from a group consisting of :Gynura divaricata subsp. formosana (Asteraceae) (GD), Murdannia bracteata (C. B. Clarke) J. K. Morton ex D. Y. Hong (Commelinaceae) (MB), and Crassocephalum rabens S. Moore (Asteraceae) (CR) with a series of solvents. A pharmaceutical, nutritional, or healthcare composition for protecting or treating acute fulminant hepatitis, for protecting or treating sepsis or related indication thereof, and a composition for skin whitening are also provided herein. These compositions all comprise effective amounts of the galactolipids-enriched plant extracts or purified compounds thereof as bioactive ingredients.
Owner:ACAD SINIC
Remedy for acute hepatitis or preventive/remedy for fulminant hepatitis
In the case where acute hepatitis progresses to fulminant hepatitis, a large amount of hepatocytes are rapidly broken and, in its turn, the prognosis is seriously worsened. Thus, it is meaningful to predict the progress of acute hepatitis into fulminant hepatitis at the early stage and quickly start an appropriate treatment therefor. Although the prediction of fulminant hepatitis becomes possibleowing to the recent advances in test methods and diagnostic techniques, there has been no appropriate preventive / remedy for fulminant hepatitis. Under these circumstances, it is intended to provide aremedy for acute hepatitis or a preventive / remedy for fulminant hepatitis with little side effect. ¢MEANS FOR SOLVING PROBLEMS! A medicinal composition for solving the above problem which contains apolipoprotein A-II.
Owner:CHUGAI PHARMA CO LTD
A drug for preventing and treating fulminant hepatitis
ActiveCN110694069BLower serum ALT levelsReduce apoptosisDigestive systemPharmaceutical active ingredientsLiver tissueLiver necrosis
The invention relates to the technical field of medicine, in particular to a medicine for preventing and treating fulminant hepatitis. The research of the present invention shows that the NOD1 receptor agonist can significantly up-regulate the expression of A20 in the liver tissue. A20 (also known as TNFAIP3) is an anti-inflammatory signaling molecule. A20 can achieve anti-apoptotic effect by down-regulating the NF-κB signaling pathway, thereby protecting liver cells. Moreover, the NOD1 receptor agonist can also significantly reduce the serum ALT level and liver tissue cl-caspase3 level in mice with fulminant hepatitis, alleviate liver injury and hepatic cell apoptosis in mice with fulminant hepatitis. Therefore, NOD1 receptor agonists are considered to be useful in the treatment of fulminant liver injury.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
Chinese medicinal composition for treating hepatitis end-stage diseases and preparation method thereof
InactiveCN101732666BImprove self-coordinationFunction increaseDigestive systemMammal material medical ingredientsButtocksEnd stage disease
The invention discloses a new Chinese medicinal composition for treating hepatitis end-stage diseases and a preparation method thereof. The Chinese medicinal composition comprises the following raw materials: dried orange peel, radix curcumae, immature bitter orange, slender dutchmanspipe root, indigowoad leaf, nutgrass galingale rhizome, szechwan chinaberry fruit, combined spicebush root, goldenthread, wild chrysanthemum, dandelion, Chinese thorowax root, houttuynia cordata thumb, bezoar, redroot gromwell root, kudzuvine root, raw gypsum, rhizoma anemarrhenae, glabrous greenbrier rhizome, weeping forsythiae capsule, double blossom, mongolian snakegourd root, lightyellow sophora root, rhubarb, Chinese dwarf cherry seed, oriental waterplantain rhizome, longhairy antenoron herb, giant knotweed rhizome, longstamen onion bulb, malt, yanhusuo, peach seed, safflower, leonurus heterophyllus, danshen root and chaff flower root. The Chinese medicinal composition can be prepared into any common oral preparations by a conventional Chinese medicinal preparation method. The Chinese medicinal composition can obviously improve symptoms such as debilitation, appetite reduction, nausea, sickness,hepatauxe and liver damage of people with last-stage hepatitis, and aundice and fever of some patients, can obviously improve symptoms such as gum errhysis, nosebleed, skin ecchymosis, hematochezia, hematemesis, hematuria, purpura occurring in lower limbs and buttocks, bloody pleural effusion or ascites and the like of patients with chronic hepatitis or last-stage fulminant hepatitis, and is characterized by exact clinical effects, obvious treatment effect and quick response. Because medicinal and edible medicaments stipulated by National Formulary are basically adopted for combination, the Chinese medicinal composition has the advantages of low cost, and no toxic or side effect.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Nucleic acid and gene originating in novel hcv strain and replicon-replicating cell using the gene
InactiveCN1882690AExcellent probabilistic replication efficiencyHigh Probability Replication EfficiencySsRNA viruses positive-senseVectorsRNA transfectionVirus strain
The present invention relates to a gene derived from a novel fulminant hepatitis C virus strain, an HCV replicon RNA with a high replication efficiency obtained using the gene, and an HCV replicon-replicating cell transfected with the replicon RNA. When the HCV replicon RNA and the HCV replicon-replicating cell of the present invention are used, HCV proteins can be continuously produced in a large amount.
Owner:TOKYO METROPOLITAN INST OF MEDICAL SCI +1
Galactolipids-enriched plant extracts and the uses thereof
The present invention is related to a galactolipids-enriched plant extract, prepared by extracting a plant sample selected from a group consisting of: Gynura divaricata subsp. formosana (Asteraceae) (GD), Murdannia bracteata (C. B. Clarke) J. K. Morton ex D. Y. Hong (Commelinaceae) (MB), and Crassocephalum rabens S. Moore (Asteraceae) (CR) with a series of solvents. A pharmaceutical, nutritional, or healthcare composition for protecting or treating acute fulminant hepatitis, for protecting or treating sepsis or related indication thereof, and a composition for skin whitening are also provided herein. These compositions all comprise effective amounts of the galactolipids-enriched plant extracts or purified compounds thereof as bioactive ingredients.
Owner:ACAD SINIC
Acute hepatic insufficiency depressant and method for evaluating drug efficacy thereof
ActiveUS9272018B2Constant effectPoor prognosisPeptide/protein ingredientsHepatocyte-growth/scatter/tumor-cytotoxic factorHepatic comaDepressant
The present invention provides a therapeutic agent for acute liver failure containing a hepatocyte growth factor (HGF), particularly an agent for treating fulminant hepatitis or late onset hepatic failure or suppression of progression of acute liver failure without hepatic coma to fulminant hepatitis or late onset hepatic failure. The present invention also provides a method for evaluating the efficacy of HGF including measuring the amount of α-fetoprotein (liver regeneration biomarker) and / or soluble Fas (anti-apoptotic biomarker) in a sample obtained from a liver injury patient administered with HGF.
Owner:KYOTO UNIV
SPARC (secreted protein, acidic and rich in cysteine), a new target for the treatment and prevention of acute liver failure
Owner:INIS BIOTEC LLC +3
Composition for preventing or treating inflammatory disease
A composition for preventing or treating inflammatory disease and that is effective for inflammatory disease such as fulminant hepatitis and interstitial pneumonia. For such an objective, the present uses a culture supernatant obtained by culturing dental pulp stem cells as the active ingredient of the composition for preventing or treating inflammatory disease.
Owner:SHED TECH CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com