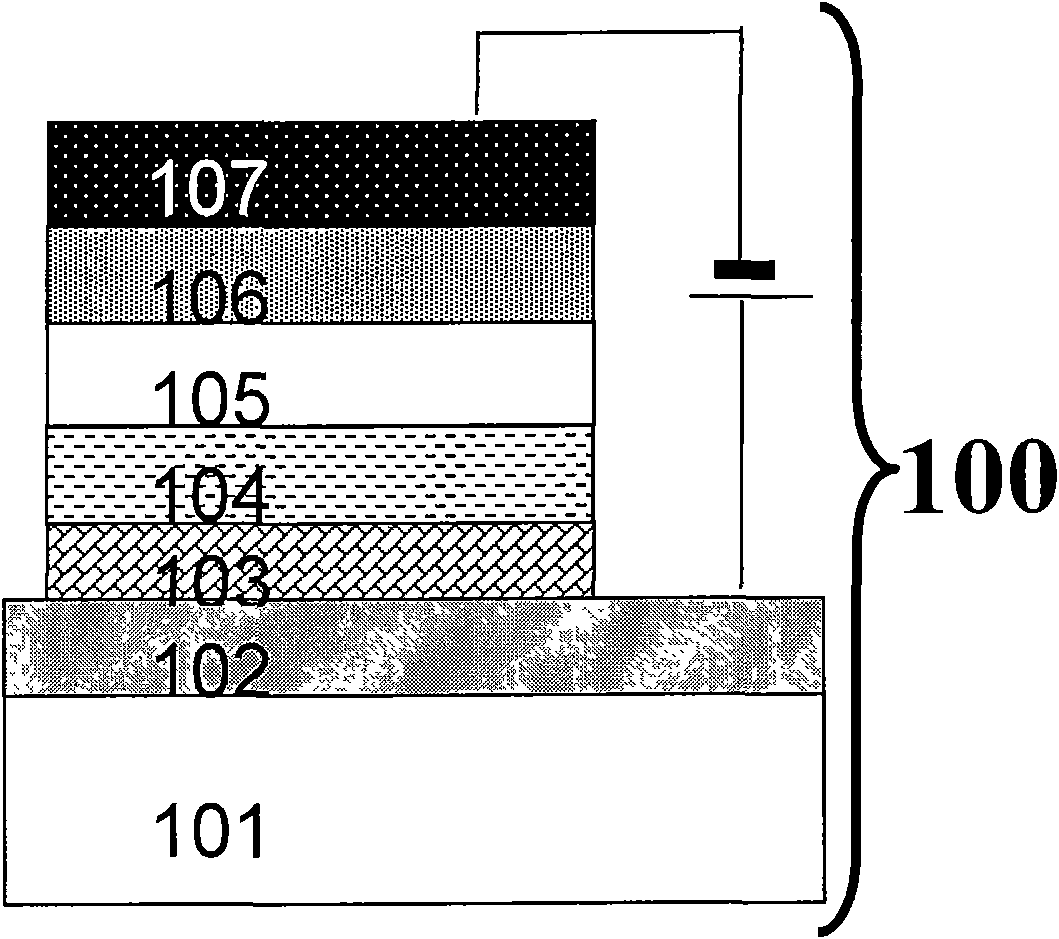
Preparation and application of N,N'-di(triphenylamine group) fluorene diamine hole injection material
A technology of hole injection layer and phenyl group, which is applied in the field of N, can solve problems such as the influence of hole injection ability, the high price of PEDOT, the reduction of the barrier between the anode and the hole transport layer, and the driving voltage of the device.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: BUHI3 described in the present invention can be synthesized by the following method.
[0067] Under nitrogen protection, 2,7-bis[N,N-bis(4-bromophenyl)]amino-9,9-di-n-butylfluorene (1.86g, 2.0mmol) was successively added to a 100mL reaction flask, Diphenylamine (2.04g, 12.0mmol), sodium tert-butoxide (1.54g, 16.0mmol), and 40mL of anhydrous toluene were added after replacing the gas in the reaction flask with inert gas. Under stirring, the pre-prepared Pd(dba) 2 (Di-p-benzylacetone 92mg, 0.16mmol), P(t-Bu) 3 (33 mg of tri-tert-butylphosphonium, 0.16 mmol) in anhydrous toluene (3 mL) was quickly added to the reaction flask, and stirred at 90° C. for 12 h. After the reaction mixture was cooled to room temperature, ammonium chloride solution was added and extracted twice with toluene. The organic phases were combined, washed with saturated brine and dried over anhydrous sodium sulfate. Part of the solvent was evaporated under reduced pressure, and the remaini...
Embodiment 2
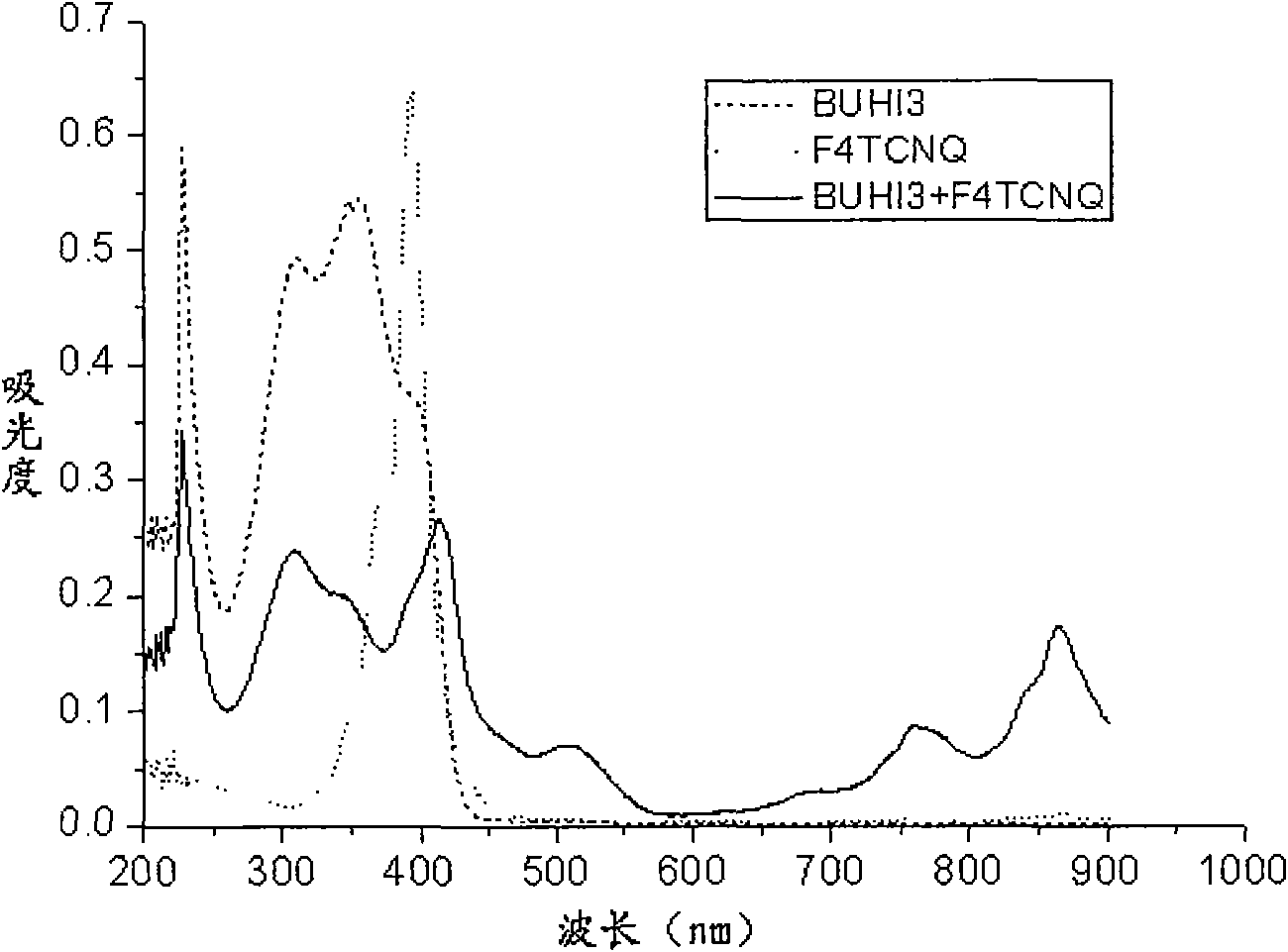
[0069] BUHI3(CHCl 3 ), F4-TCNQ (CHCl 3 ), BUHI3+F4-TCNQ (CHCl 3 ) UV-Vis see image 3 . For the cyclic voltammetry experiment of BUHI3, see Figure 4 . The highest occupied electron orbital (HOMO) of the host material molecule BUHI3 forming the P-type doping system is 5.07eV, and the lowest unoccupied orbital (LUMO) of the dopant molecule F4-HCNQ is 5.24eV. The energy levels of the two are similar, and the charge transfer occurs. Increased conductivity for hole transport.
Embodiment 3
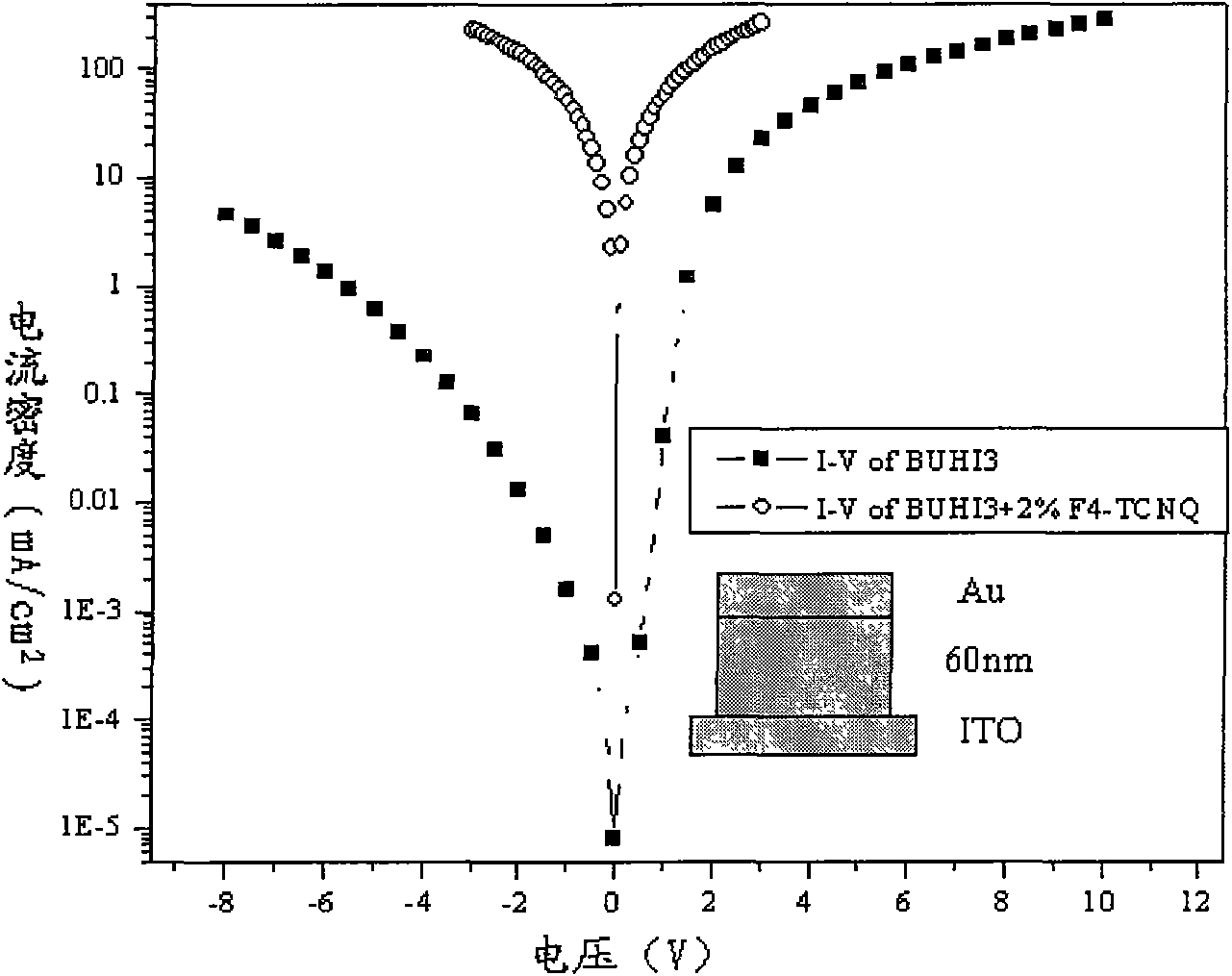
[0071] This example demonstrates pure hole transport devices fabricated from BUHI3 and BUHI3 doped with F4-TCNQ, respectively. The ITO glass was ultrasonically cleaned in detergent and deionized water successively for 30 minutes. Then vacuum dry for 2 hours (120° C.), then treat the ITO glass with UV / ozone for 25 minutes, and send it to the vacuum chamber to prepare organic film and metal electrode. This experiment includes two devices, the structures are: device 1: ITO / BUHI3(60nm) / Au; device 2: ITO / BUHI3+2%F4-TCNQ(60nm) / Au, where ITO is the square resistance of 10-20 ohms Transparent electrode, BUHI3 is a hole transport semiconductor, and F4-TCNQ is a doping material. Depend on figure 2 It can be seen that after BUHI3 is doped with F4-TCNQ, the device has good hole injection and conductivity, and its current density is two orders of magnitude higher than that of device 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com