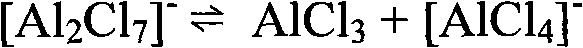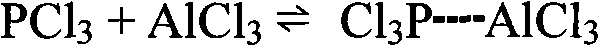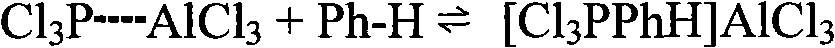Environmentally-friendly synthesis method for phenylphosphonic dichloride
A technology of phenylphosphine dichloride and phosphorus trichloride, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, chemical recovery, etc. Regeneration, inability to directly separate and other problems, to achieve obvious environmental protection, low production costs, less product by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Ionic liquid [BuPy]Cl-AlCl 3 Preparation of (BuPy=N-butylpyridinium cation)
[0038] Install a stirrer on the reaction kettle, add 26.67g (0.2mole) of anhydrous AlCl under the protection of nitrogen 3 , added in batches (0.1 mole) n-butylpyridine chloride. After adding the quaternary ammonium salt, keep stirring at about 60°C for 3 hours to ensure the completion of the reaction, and obtain a transparent light brown [BuPy]Cl-AlCl 3 ionic liquid.
[0039] (2) Preparation of phenylphosphine dichloride
[0040] Install a stirrer and a hydrogen chloride absorption system on the reaction kettle, add the ionic liquid prepared above, 78g (1 mole) benzene, and 411.9g (3 mole) phosphorus trichloride, heat to reflux, and keep the reflux reaction for 18 hours. After the reaction is finished, cool to room temperature and let it stand. The solution is divided into two layers. The brown liquid in the lower layer is the ionic liquid layer, and the upper layer is a colorless c...
Embodiment 2
[0043] The reclaimed ionic liquid described in Example 1 is used instead of the catalyst used in Example 1, and phenylphosphine dichloride is prepared according to the method in Example 1. The yield in terms of benzene is 64%, and product analysis shows that phosphorus trichloride is contained in the product 1.2%, phenylphosphine dichloride 98.8%, the product does not contain other impurities.
Embodiment 3
[0045] (1) Ionic liquid [trEHAm]Cl-AlCl 3 Preparation of (trEHAm = hydrogenated triethylamine cation)
[0046]Install a stirrer on the reaction kettle, add 26.67g (0.2mole) of anhydrous AlCl under the protection of nitrogen 3 , Add 13.8g (0.1mole) triethylamine hydrochloride in batches. After adding the quaternary ammonium salt, keep stirring at about 120°C for 2 hours to ensure the completion of the reaction, and obtain a transparent and colorless [trEtHAm]Cl-AlCl 3 ionic liquid.
[0047] (2) Preparation of phenylphosphine dichloride
[0048] Install a stirrer and a hydrogen chloride absorption system on the reactor, add the ionic liquid prepared above, 78g (1mole) benzene, and 411.9g (3mole) phosphorus trichloride, heat to reflux, and keep the reflux reaction for 18 hours. After the reaction, cool to room temperature and let it stand, the solution is divided into two layers, the lower layer is a colorless transparent liquid, mainly containing unreacted benzene, phosphoru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com