a drug for diabetes
A technology for diabetes and drugs, applied in the field of drugs for the treatment of diabetes, to achieve the effect of enhancing insulin sensitivity, simple process, and reducing fasting and postprandial blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the preparation of the medicine for the treatment of diabetes
[0027] With 1 mass part of scorpion crude drug (Shenzhen Lifeng Pharmaceutical Co., Ltd., batch number 20090314) and 10 mass parts of gypsum crude drug (the main component is CaSO 4 2H 2 O, Shenzhen Lifeng Pharmaceutical Co., Ltd., batch number 20090118) prepares the medicine for the treatment of diabetes (the above Chinese herbal medicines have all reached the corresponding quality control detection standard established by the 2005 edition of the Pharmacopoeia of the People's Republic of China (Part One)), the steps are as follows:
[0028] 1. Dry the scorpion at 60°C for 24 hours, crush it, and pass it through a 100-mesh sieve to get the scorpion powder;
[0029] 2. Add 5 times the weight of distilled water to the gypsum, bathe in 95°C water for 1 hour, and filter with cotton or gauze to obtain the gypsum water extract (suspension);
[0030] 3. The scorpion powder and the water extract of ...
Embodiment 2
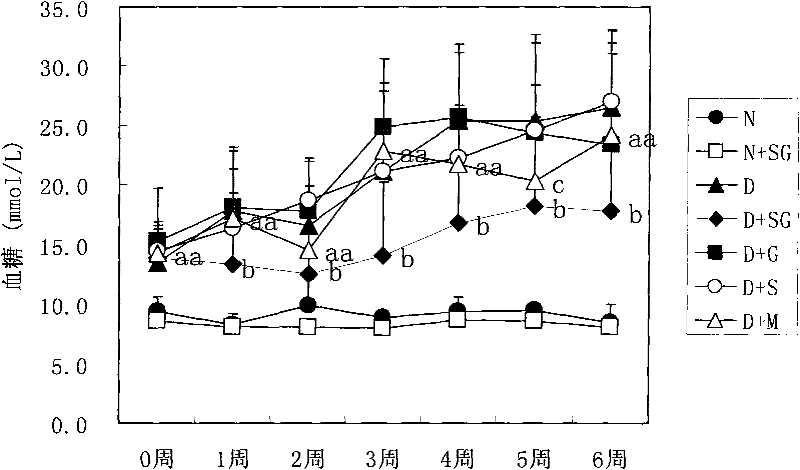
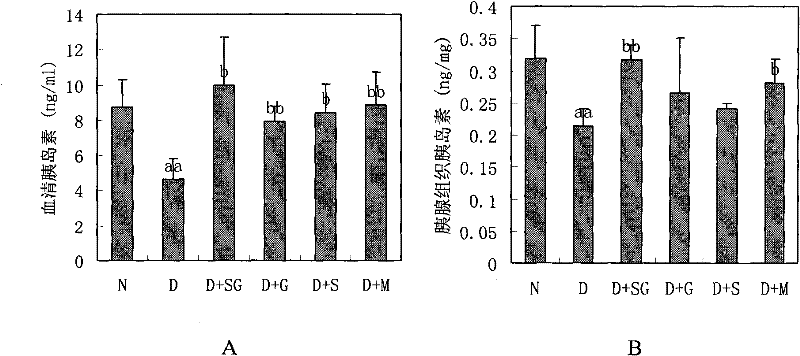
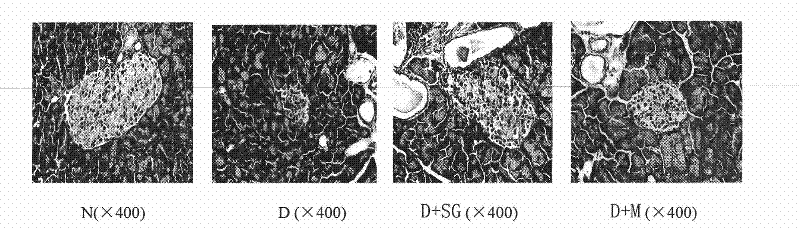
[0031] Embodiment 2, the drug effect of the medicine for the treatment of diabetes
[0032] 1. Production of NIH mouse diabetes model
[0033] NIH male mice (about 20 g) were fasted for 24 hours, and injected intraperitoneally once with 100 mg / kg streptozotocin (Streptozotocin, Sigma Company, USA) (streptozotocin was freshly prepared with ice-bathed citrate buffer solution in advance). reconstituted (0.1M, pH 4.5), injected within 5 minutes). After 10 days, the mice with a stable increase in blood sugar (blood sugar above 11.1 mmol / L) were the NIH mouse diabetes models.
[0034] 2. Administration of NIH mouse diabetes model
[0035] Administration group A (scorpion treatment group; D+S): NIH mouse diabetes model; the scorpion powder of step 1 of Example 1; 350mg scorpion powder / kg mouse / day;
[0036] Administration group B (gypsum treatment group; D+G): NIH mouse diabetes model; the water extract of gypsum in step 2 of Example 1; the water extract of 3.5g gypsum / kg mouse / day;...
Embodiment 3
[0070] Embodiment 3, the glucose tolerance experiment of mice after administration
[0071] 1. Oral glucose tolerance
[0072] 1. Production of NIH mouse diabetes model
[0073] Same as Step 1 of Example 2.
[0074] 2. Administration of NIH mouse diabetes model
[0075] Scorpion+gypsum treatment group (D+SG): NIH mouse diabetes model; the medicine for treating diabetes in the step 3 of embodiment 1; (the medicine obtained by mixing the water extract of 350mg scorpion powder and 3.5g gypsum) / kg mouse / day;
[0076] Metformin group (D+M): NIH mouse diabetes model; Metformin (Tianjin Pacific Pharmaceutical Technology Group, production batch number 071101); 250mg metformin / kg mouse / day;
[0077] Normal group (N): normal NIH male mice; double distilled water;
[0078] Diabetic control group (D): NIH mouse diabetes model; double distilled water;
[0079]In the above administration groups, the drug was suspended in equal volume of double distilled water before use, and administ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com