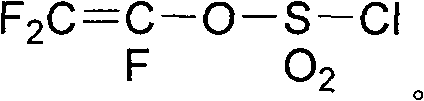
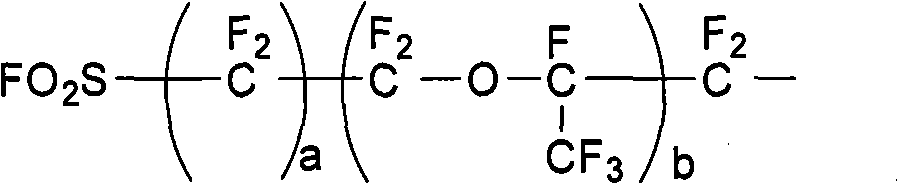Method for preparing fluoro olefin
A technology of fluoroolefins and fluorovinyl sulfate, applied in the field of fluorine-containing fine chemicals, can solve the problems of low monomer yield, complicated preparation process, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
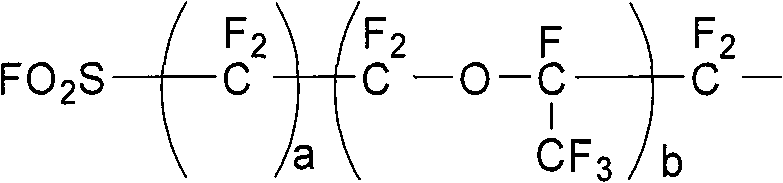
[0028] Clean and fully dry the 10L stainless steel high-pressure reactor equipped with a circulating cooling / heating system, temperature control system, and feeding system, then vacuumize it, fill it with nitrogen and replace it three times until the oxygen content is controlled below 10ppm, and then vacuumize it to -0.1MPa. Add 760g cesium fluoride, 580g potassium fluoride, solvent tetraethylene glycol dimethyl ether 2Kg, trifluorovinyl sulfate 1965g in the system, solvent consumption is 101.8% of trifluorovinyl sulfate total mass, catalyst consumption The number of moles is 1.5 times of the total molar amount of trifluorovinyl sulfate added, and the stirring temperature is controlled at 25°C, and tetrafluorosultone (molecular formula: FO 2 SCF 2 COF) 1800g, just keep the temperature at 25±1°C. After the dropwise addition, stir at constant temperature for two hours, stop stirring, lower the temperature, let it stand for stratification, and pass the lower layer of colorless an...
Embodiment 2
[0031] Clean and fully dry the 10L stainless steel high-pressure reactor equipped with a circulating cooling / heating system, temperature control system, and feeding system, then vacuumize it, fill it with nitrogen and replace it three times until the oxygen content is controlled below 10ppm, and then vacuumize it to -0.1MPa. In the system, add 304g cesium fluoride, 580g potassium fluoride, solvent adiponitrile 2Kg, trifluorovinyl sulfate 1965g, solvent consumption is 101.8% of trifluorovinyl sulfate total mass, and the mole number of catalyst consumption is Add 1.2 times the total molar amount of trifluorovinyl sulfate, stir and control the temperature at 15°C, slowly add the monoaddition product of tetrafluorosultone and hexafluoropropylene oxide (molecular formula: FO 2 SCF 2 CF 2 OCF (CF 3 ) COF) 3460g, the temperature can be controlled at 15±1°C. After the dropwise addition is completed, stir at a constant temperature for two hours, stop stirring, cool down, stand for st...
Embodiment 3
[0034] Clean and fully dry the 10L stainless steel high-pressure reactor equipped with a circulating cooling / heating system, temperature control system, and feeding system, then vacuumize it, fill it with nitrogen and replace it three times until the oxygen content is controlled below 10ppm, and then vacuumize it to -0.1MPa. Add 608g cesium fluoride, 522g potassium fluoride into the system, solvent tetraethylene glycol dimethyl ether 1Kg, acetonitrile 1Kg, trifluorovinyl sulfate 1965g, solvent consumption is 101.8% of the total mass of trifluorovinyl sulfate %, the molar number of the catalyst consumption is 1.3 times of the total molar amount of the added trifluorovinyl sulfate ester, stirring and temperature control is at 10 ℃, slowly dripping 3-acyl fluoride-propanesulfonyl fluoride ( Molecular formula: FO 2 SCF 2 CF 2 COF) 2300g, the temperature can be controlled at 10±1°C. After the dropwise addition is completed, stir at a constant temperature for two hours, stop stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com