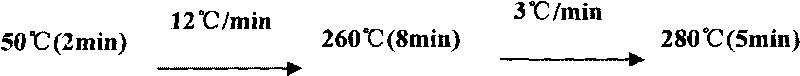Derivatization method of environmental estrogens
An environmental estrogen and derivatization technology, applied in the field of derivatization of environmental estrogen, can solve the problems of increasing energy consumption and cost of derivatization experiments, reducing detection accuracy, increasing sample loss, etc., and simplifying the derivatization reaction system. , The effect of reducing the experimental cost and reducing the loss of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
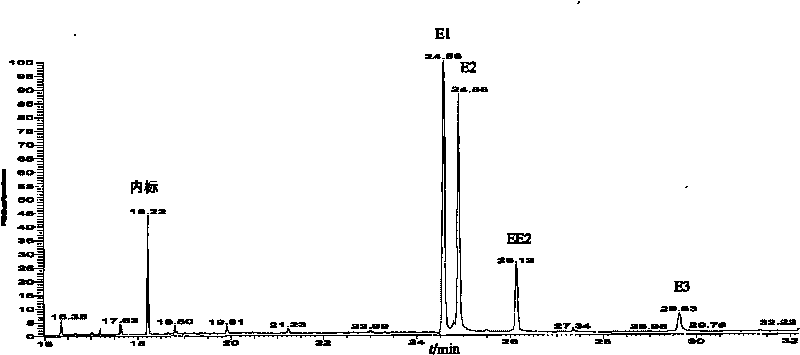
[0038] 1. First, take 100 μL of the standard mixed solution containing a certain concentration (10pg / μL) of E1, E2, EE2, and E3 in a 1.5mL chromatographic bottle, put the chromatographic bottle into a nitrogen blower, and blow it dry slowly with high-purity nitrogen.
[0039] 2. Add 15 μL of BSTFA and 30 μL of pyridine, shake and mix, and place in a normal temperature environment (20° C.) for 3 minutes to react.
[0040] 3. Take it out and cool it to room temperature, put it into a nitrogen blower, blow it dry slowly with high-purity nitrogen, add 90 μL of injection solvent and 10 μL of internal standard (androstane) with a concentration of 1 ng / μL, shake and mix, take 1 μL and inject GC-MS analysis (SIM mode).
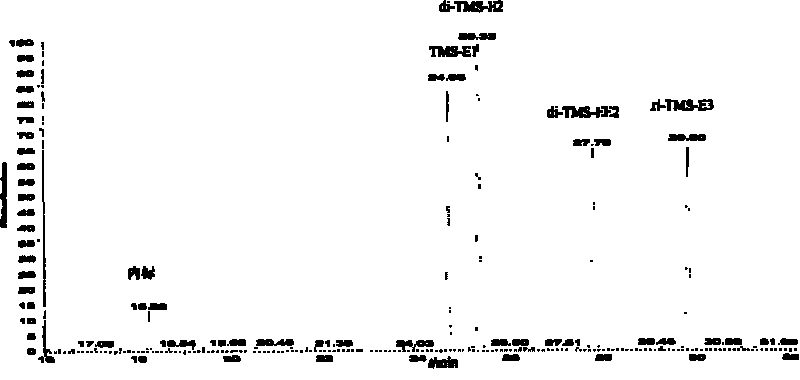
[0041] After testing, E1, E2, EE2, and E3 have all been derivatized, and the derivatized products are TMS-E1, di-TMS-E2, di-TMS-EE2, tri-TMS-E3, no by-products are generated, RRF( The relative response factor RelativeResponse Factor, the ratio of the peak area of t...
Embodiment 2
[0043] 1. First, take 100 μL of the standard mixed solution containing a certain concentration (10ng / μL) of E1, E2, EE2, and E3 in a 1.5mL chromatographic bottle, put the chromatographic bottle into a nitrogen blower, and blow it dry slowly with high-purity nitrogen.
[0044] 2. Add 30 μL of BSTFA and 50 μL of pyridine, shake and mix, and place in a room temperature environment (20° C.) to react for 5 minutes.
[0045] 3. Take it out and cool it to room temperature, put it into a nitrogen blower, blow it dry slowly with high-purity nitrogen, add 90 μL of injection solvent and 10 μL of internal standard (androstane) with a concentration of 1 ng / μL, shake and mix, take 1 μL and inject GC-MS analysis (SIM mode).
[0046] After testing, E1, E2, EE2, and E3 have all been derivatized, and the derivatized products are TMS-E1, di-TMS-E2, di-TMS-EE2, tri-TMS-E3, respectively, and no by-products are generated. For 1157.76, 1074.82, 146.65, 187.13.
Embodiment 3
[0048] 1. First, take 100 μL of the standard mixed solution containing a certain concentration (8pg / μL) of E1, E2, EE2, and E3 in a 1.5mL chromatographic bottle, put the chromatographic bottle into a nitrogen blower, and blow it dry slowly with high-purity nitrogen.
[0049] 2. Add 15 μL of BSTFA and 20 μL of pyridine, shake and mix, put into a constant temperature heating box, and react at 30°C for 3 minutes.
[0050] 3. Take it out and cool it to room temperature, put it into a nitrogen blower, blow it dry slowly with high-purity nitrogen, add 90 μL of injection solvent and 10 μL of internal standard (androstane) with a concentration of 1 ng / μL, shake and mix, take 1 μL and inject GC-MS analysis (SIM mode).
[0051] After testing, E1, E2, EE2, and E3 have all been derivatized, and the derivatized products are TMS-E1, di-TMS-E2, di-TMS-EE2, tri-TMS-E3, respectively, and no by-products are generated. 0.99, 0.88, 0.06, 0.08.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com