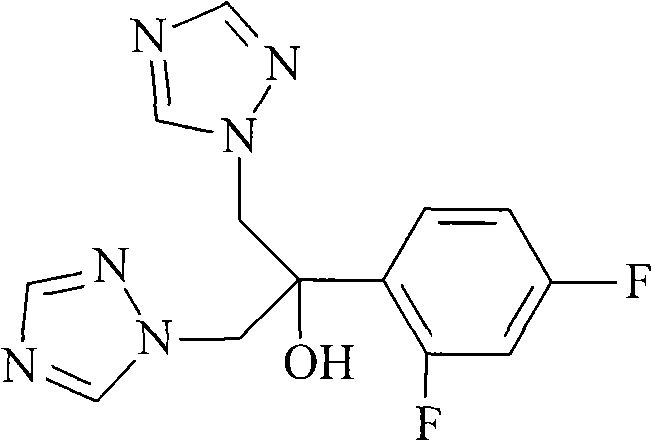
Solid preparation of fluconazole liposome and new application thereof
A technology of fluconazole lipid and solid preparation, applied in the field of liposome preparation, can solve the problems of low dissolution rate, poor stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
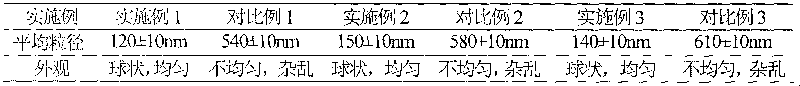
Embodiment 1
[0072] The preparation of embodiment 1 fluconazole liposome
[0073] prescription:
[0074] Component Dosage
[0075] Fluconazole 25g
[0076] Soy Lecithin 55g
[0077] Cholesterol 32.5g
[0078] Ascorbyl Palmitate 2.5g
[0079] Preparation Process
[0080] (1) 25g fluconazole, 55g soybean lecithin, 32.5g cholesterol and 2.5g ascorbyl palmitate are dissolved in the mixed solvent of methanol, Virahol and acetone with a volume ratio of 2:1:2 in 600ml, mix homogeneously, Remove the mixed solvent under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0081] (2) Add 450ml of citric acid-sodium citrate buffer solution with a pH value of 6.5, shake and stir for 20 minutes at a speed of 600r / min to completely hydrate the phospholipid membrane, and emulsify it at a high speed using a tissue masher for 20 minutes minutes, keep warm at 60°C, rotate at 12000r / min, and then filter with a 0.45 μm microporous membrane to obtain a liposome suspension; ...
Embodiment 2
[0083] The preparation of embodiment 2 fluconazole liposomes
[0084] prescription:
[0085] Component Dosage
[0086] Fluconazole 50g
[0087] Soy Lecithin 210g
[0088] Cholesterol 150g
[0089] Ascorbyl Palmitate 22.5g
[0090] Preparation Process
[0091] (1) 50g fluconazole, 210g soybean lecithin, 150g cholesterol and 22.5g ascorbyl palmitate are dissolved in the mixed solvent of ethanol, isopropanol and methanol in a volume ratio of 1:1:2 in 3400ml, mix well, and The mixed solvent was removed under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0092] (2) Add 2000ml of phosphoric acid-sodium hydrogen phosphate buffer solution with a pH value of 5.5, shake, stir for 40 minutes, and rotate at a speed of 200r / min to completely hydrate the phospholipid film, and use a tissue masher for high-speed homogeneous emulsification for 10 minutes, Insulate at 50°C, rotate at 15000r / min, and then filter with a 0.45μm microporous membrane to o...
Embodiment 3
[0094] The preparation of embodiment 3 fluconazole liposomes
[0095] prescription:
[0096] Component Dosage
[0097] Fluconazole 100g
[0098] Soy Lecithin 600g
[0099] Cholesterol 450g
[0100] Ascorbyl Palmitate 80g
[0101] Preparation Process
[0102] (1) 100g fluconazole, 600g soybean lecithin, 450g cholesterol and 80g ascorbyl palmitate are dissolved in the mixed solvent of tert-butanol, acetone and methanol in a volume ratio of 3:2:2 in 7500ml, mix well, and The mixed solvent was removed under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0103] (2) Add 3500ml of citric acid-sodium citrate buffer solution with a pH value of 7.5, shake, stir for 30 minutes, and rotate at 400r / min to completely hydrate the phospholipid film, and use a tissue masher for high-speed homogeneous emulsification for 15 minutes Minutes, keep warm at 60°C, rotate at 15000r / min, and then filter with a 0.45 μm microporous membrane to obtain a liposome ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com