Porous bone replacing material and preparation method thereof
A porous bone and chitosan technology, applied in medical science, prosthesis, etc., can solve problems such as difficulty in maintaining antibacterial concentration, lack of porous materials, and uncontrollable slow release of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for preparing a porous bone substitute material, the specific steps are:
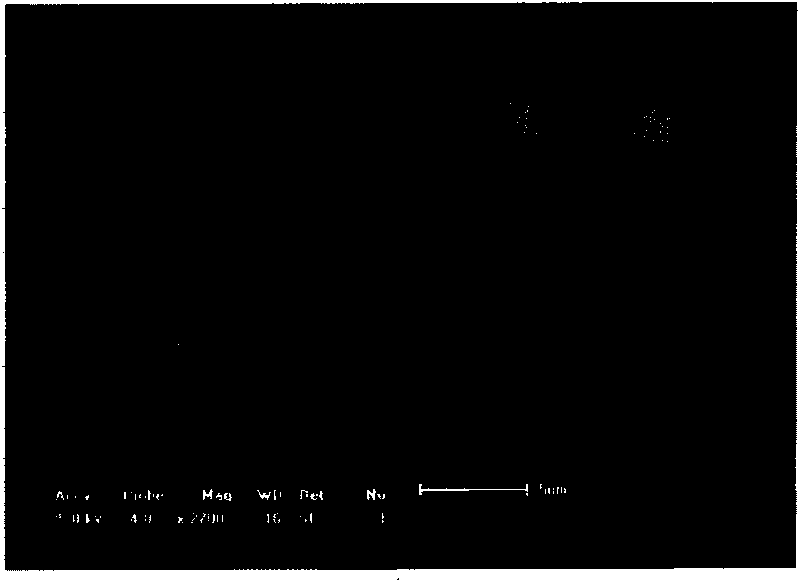
[0029] The first step: dissolving chitosan in 1wt% glacial acetic acid solution to obtain chitosan concentration is a solution of 10g / L, vancomycin is dissolved in this solution, and the weight ratio of vancomycin and chitosan is 1: 1. Spray drying to prepare chitosan / vancomycin microspheres with a particle size of 5-15 μm, the conditions of the spray drying are: inlet temperature 160 degrees, outlet temperature 90 degrees, injection speed 20mL / min, nozzle diameter 0.75mm, injection pressure 2 atmospheres; figure 1 Shown is the schematic diagram of the chitosan / vancomycin microspheres obtained by spray drying; the chitosan / vancomycin microspheres are moved into a sodium alginate solution with a concentration of 1g / L, centrifuged, and the supernatant is discarded to obtain The chitosan / vancomycin microspheres with sodium alginate coating are 10mg according to the weight volume ratio: 1ml...
Embodiment 2
[0034] A method for preparing a porous bone substitute material, the specific steps are:
[0035] The first step: dissolving chitosan in 1wt% glacial acetic acid solution to obtain chitosan concentration is a solution of 10g / L, vancomycin is dissolved in this solution, and the weight ratio of vancomycin and chitosan is 1: 1. Spray drying to prepare chitosan / vancomycin microspheres with a particle size of 5-15 μm, the conditions of the spray drying are: inlet temperature 160 degrees, outlet temperature 90 degrees, injection speed 20mL / min, nozzle diameter 0.75mm, spray pressure 2 atmospheres; move the chitosan / vancomycin microspheres into the sodium alginate solution with a concentration of 1g / L, centrifuge, discard the supernatant, and the obtained shell with sodium alginate coating The polysaccharide / vancomycin microspheres are 10mg according to the weight volume ratio: 1ml is cross-linked in 0.1% cross-linking agent glutaraldehyde solution; the particle diameter of the chito...
Embodiment 3
[0040] A method for preparing a porous bone substitute material, the specific steps are:
[0041] The first step: dissolving chitosan in 1wt% glacial acetic acid solution to obtain chitosan concentration is a solution of 10g / L, vancomycin is dissolved in this solution, and the weight ratio of vancomycin and chitosan is 1: 1. Spray drying to prepare chitosan / vancomycin microspheres with a particle size of 5-15 μm, the conditions of the spray drying are: inlet temperature 160 degrees, outlet temperature 90 degrees, injection speed 20mL / min, nozzle diameter 0.75mm, spray pressure 2 atmospheres; move the chitosan / vancomycin microspheres into the sodium alginate solution with a concentration of 1g / L, centrifuge, discard the supernatant, and the obtained shell with sodium alginate coating The polysaccharide / vancomycin microspheres are cross-linked according to the weight-to-volume ratio of 10mg: 1ml by adding 0.1% cross-linking agent solution; the particle size of the chitosan / vanco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com