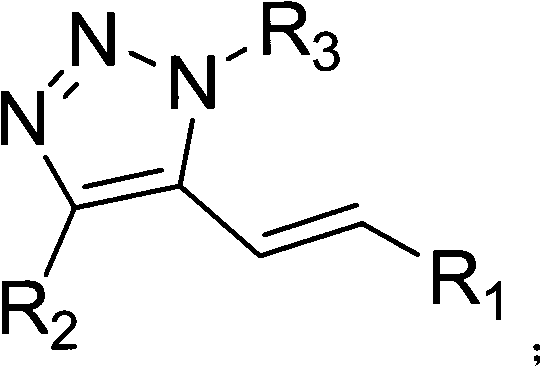
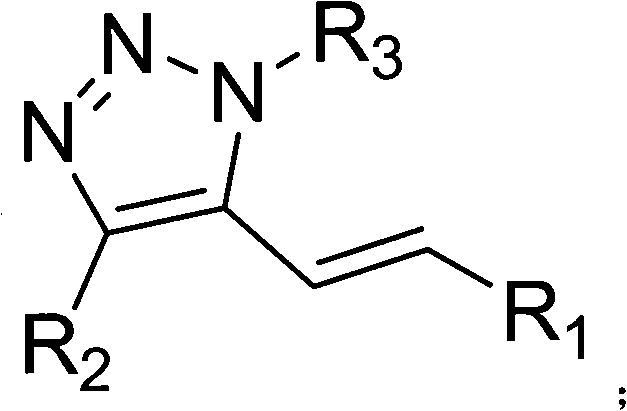
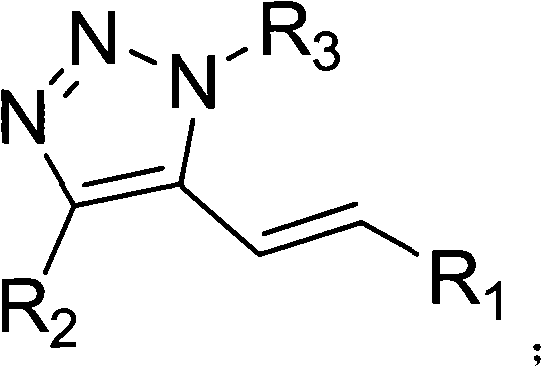
5-alkenyl-1, 4-disubstituted-1, 2, 3-triazole compound, preparation method and application thereof
A triazole compound, alkenylation technology, applied in the directions of botanical equipment and methods, applications, organic chemistry, etc., can solve the problems of expensive raw materials, complicated steps, complicated synthesis steps, etc., and achieves the advantages of industrial production, simple and easy steps. Good effect of line and reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In the autoclave, add 2ml of 1,4-dioxane, 1mmol of copper acetate, 2mmol of sodium acetate, 0.05mmol of palladium acetate, 0.5mmol of 1-octyl-4-phenyl-1,2 , 3-triazole and 1 mmol of methyl acrylate, filled with 0.8MPa oxygen, and stirred at 110°C for 24 hours. After the reaction, the autoclave system was cooled to 0°C, and the system was slowly degassed to extract and separate the product. Column chromatography was used for further separation and purification to obtain a product with a purity of more than 99%. The column chromatography condition used was petroleum ether:ethyl acetate with a volume ratio of 20:1, and the yield was 87%.
Embodiment 2
[0036]In the autoclave, add 3ml of 1,4-dioxane, 1mmol of copper acetate, 2mmol of sodium acetate, 0.05mmol of palladium chloride, 0.5mmol of 1-octyl-4-phenyl-1, 2,3-triazole and 1 mmol of methyl acrylate were filled with 0.2 MPa oxygen, and stirred at 90°C for 20 hours. After the reaction, the autoclave system was cooled to 0°C, and the system was slowly degassed to extract and separate the product. Column chromatography was used for further separation and purification to obtain a product with a purity of more than 99%. The column chromatography condition used was petroleum ether:ethyl acetate with a volume ratio of 20:1, and the yield was 3%.
Embodiment 3
[0038] In the autoclave, add 3ml of 1,4-dioxane, 1mmol of copper acetate, 2mmol of potassium carbonate, 0.05mmol of palladium nitrate, 0.5mmol of 1-octyl-4-phenyl-1,2 , 3-triazole and 1mmol of methyl acrylate, filled with 1.2MPa oxygen, stirred and reacted at 160°C for 48h, after the reaction was completed, the autoclave system was cooled to 0°C, the system was slowly degassed, and the product was extracted and separated. Chromatography was further separated and purified to obtain a product with a purity of more than 99%. The column chromatography used was petroleum ether:ethyl acetate with a volume ratio of 20:1, and the yield was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com