Method for separating sodium fluoride crystals from silicon dioxide
A silica and separation method technology, applied in the direction of alkali metal fluoride, silicate, alkali metal silicate, etc., can solve the problems such as the difficulty of separating sodium fluoride and silica, achieve small investment and improve utilization rate, reduce the effect of process loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
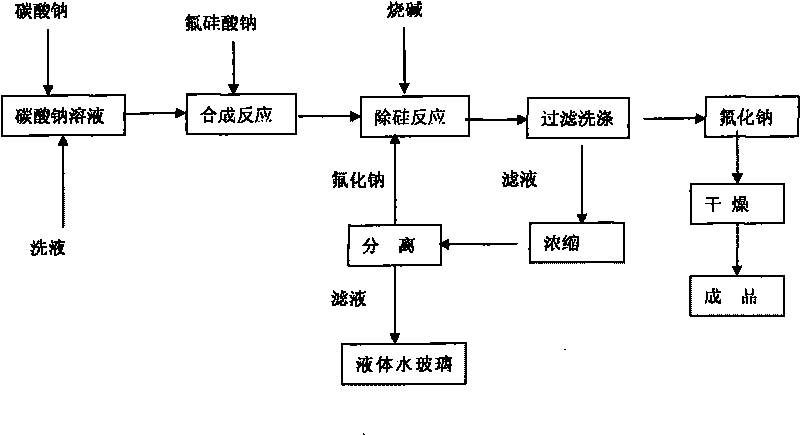
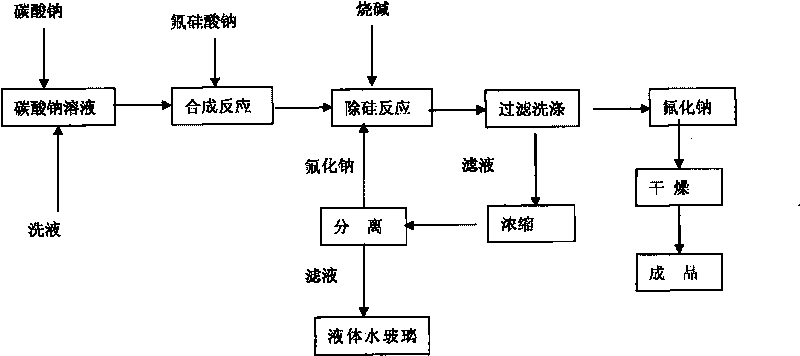
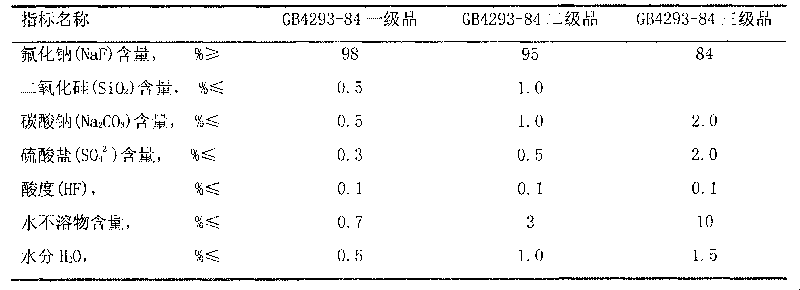
[0022] Equipped with a heating device, a stirring device, and a concentrating device, weigh 482g of solid sodium carbonate, process 1100g of water to prepare a 30% solution, raise the temperature to 85°C, press Na 2 SiF 6 :Na 2 CO 3 =1:1.05~1.1 For batching, slowly add 400 g of solid sodium fluorosilicate for 30 minutes, and continue to react at 93° C. for 30 minutes. Add sodium hydroxide solution, the amount of NaOH added is 35% of the theoretical amount in molar ratio, weigh 58g of solid NaOH, and prepare a 50% solution, slowly add to the above solution, and continue to react for 30 minutes. Filter and wash the sodium fluoride filter cake with 600g of hot water to obtain a wet filter cake and dry it in an oven at 120°C for 2 hours to obtain 494g of finished sodium fluoride with a product yield of 92%. The standard analysis results of the product fully meet the GB4293-84 standard First class requirements.
[0023] The filtrate contains SiO 2 11.46%, Na 2 O 4.83%, densi...
Embodiment 2
[0025] Equipped with a heating device, a stirring device, and a concentrating device, weigh 482g of solid sodium carbonate, process 1100g of water to prepare a 30% solution, raise the temperature to 85°C, press Na 2 SiF 6 :Na 2 CO 3 =1:1.05 Dosing, slowly add 400g of solid sodium fluorosilicate, the addition time is 30 minutes, and continue to react at 93°C for 30 minutes. Add sodium hydroxide solution, the amount of NaOH added is 35% of the theoretical amount in molar ratio, weigh 58g of solid NaOH, and prepare a 50% solution, slowly add to the above solution, and continue to react for 30 minutes. Filter and wash the sodium fluoride filter cake with 600g of hot water to obtain a wet filter cake and dry it in an oven at 120°C for 2 hours to obtain 494g of finished sodium fluoride with a product yield of 92%. The standard analysis results of the product fully meet the GB4293-84 standard First class requirements.
[0026] The filtrate contains SiO 2 11.46%, Na 2 O 4.83%, ...
Embodiment 3
[0028] Equipped with a heating device, a stirring device, and a concentrating device, weigh 482g of solid sodium carbonate, add 1417g of process water to prepare a 25% solution, raise the temperature to 85°C, press Na 2 SiF 6 :Na 2 CO 3 =1:1.05~1.1 For batching, slowly add 400 g of solid sodium fluorosilicate for 30 minutes, and continue to react at 93° C. for 30 minutes. Add sodium hydroxide solution, the amount of NaOH added is 40% of the theoretical amount, weigh 67g of solid NaOH, and prepare a 50% solution, slowly add to the above solution, and continue to react for 30 minutes. Filter and wash the sodium fluoride filter cake with 600g of hot water to obtain a wet filter cake and dry it in an oven at 120°C to obtain 483g of finished sodium fluoride with a product yield of 90%. The product adopts the standard analysis results to fully meet the GB4293-84 standard level one product requirements.
[0029] The filtrate contains SiO 2 12.43%, Na 2 O5.21%, density 1.192, F ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com