Method for preparing quaternary amine dual-functional ionic liquid
An ionic liquid, dual-function technology, applied in chemical instruments and methods, preparation of carboxylates, preparation of amino compounds from amines, etc., to achieve the effects of low cost of raw materials, simple synthesis route, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Ion exchange method prepares [A336][NO 3 ]
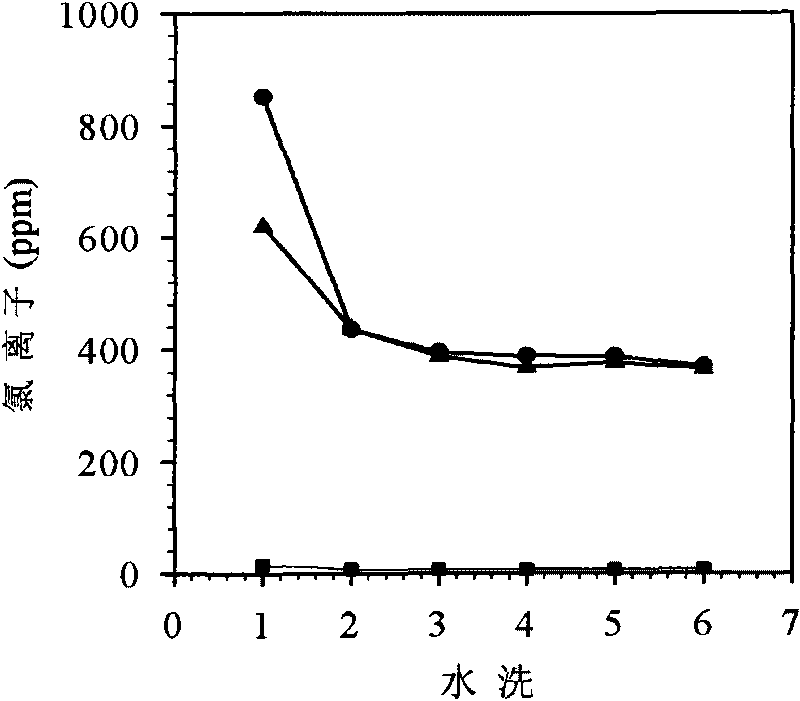
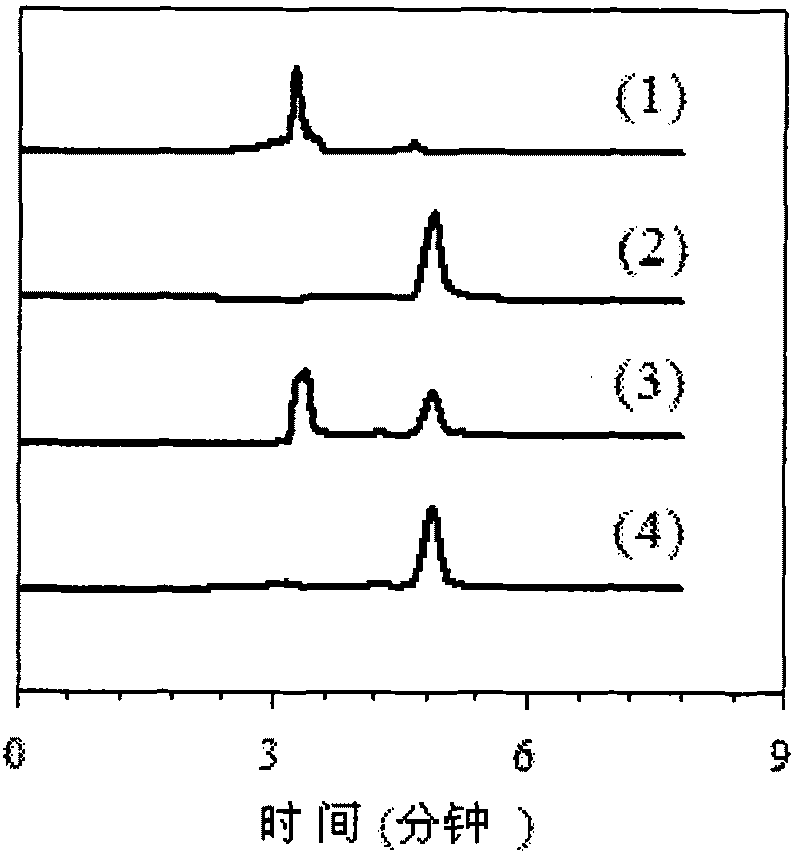
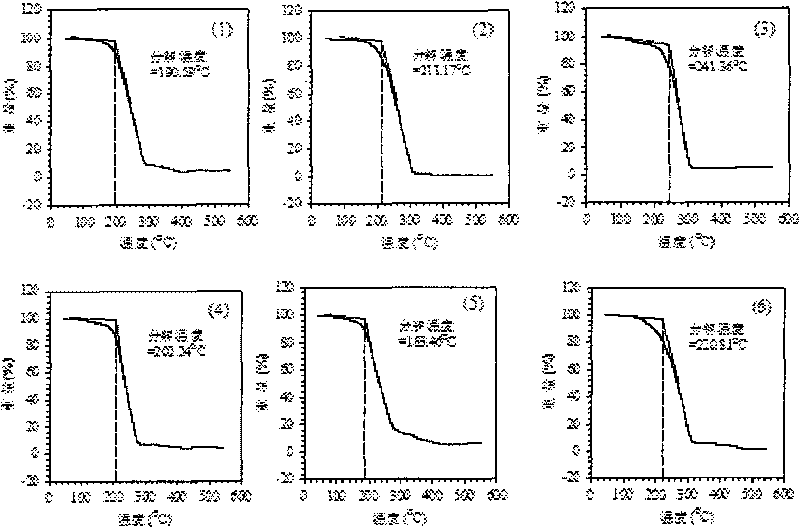
[0037] According to the steps described in the synthesis route of the ion exchange method in the technical background, 8.5 g of sodium nitrate (0.1 mol) was dissolved in 20 mL of deionized water. Then 40.457 g (0.1 mol) of Aliquat336 was dissolved in 50 mL of acetone. Under magnetic stirring, the acetone solution containing Aliquat336 was added dropwise to the sodium nitrate aqueous solution, the acetone-water solution was stirred at room temperature for 1 h, the layers were left to stand, and the upper phase was taken out. Rotate the upper phase with a rotary evaporator at 80°C for 50 minutes to spin out the isopropanol and water contained in it to obtain [A336][NO 3 ]. The above [A336][NO 3 ] was dissolved in 50 mL of acetone and the sodium chloride was filtered off with a 4.5 micron filter. Spinning for another hour removes the acetone to give [A336][NO 3 ] (structure as shown in formula 1). Income[A33...
Embodiment 2
[0038] Embodiment 2: the influence of methanol, ethanol, propanol, isopropanol on preparation [A336][OH]
[0039] Dissolve 112.36 grams of Aliquat 336 in 500 mL of distilled methanol, ethanol, propanol, and isopropanol, and dissolve them completely. Add 6.39 g of metallic sodium to 5 plastic bottles, then add 125 mL of distilled methanol, ethanol, propanol, and isopropanol, and react at room temperature for 3 hours to prepare sodium alkoxide. The above solutions were mixed and stirred at 50°C for 4 hours to prepare [A336][OR]. The resulting solution was centrifuged at 8000 rpm for 10 minutes to remove the sodium chloride precipitate. Add 500 ml of deionized water to the filtrate, shake for 30 minutes, and perform hydrolysis to prepare [A336][OH]. Take 1 ml of the above-mentioned [A336][OH], respectively, and use bromothymol blue as an indicator to titrate with hydrochloric acid with a concentration of 0.132 mol / L. It is measured that the yields of [A336][OH] prepared by met...
Embodiment 3
[0040] In order to determine the influence of different types and concentrations of acids on [A336][OH] titration, take 1 ml of the above-mentioned [A336][OH] respectively, use bromothymol blue as indicator, and use hydrochloric acid with a concentration of 0.132mol / l Titration was carried out and the result was 0.121 mol / l. Under the same conditions, titrate with nitric acid with a concentration of 0.298 mol / l, and the result is 0.123 mol / l. The results showed that different types and concentrations of acids had little effect on the titration results.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com