Oral preparation of novel colonic positioning release medicine
A technology of colon-localized and oral preparations, applied in the field of olsalazine sodium colon-localized release tablets, which can solve problems such as irritation and obvious adverse reactions of the stomach, and achieve the effect of improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
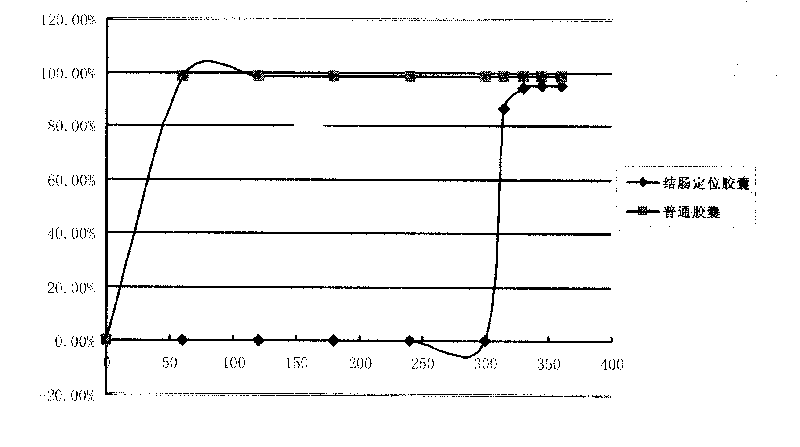
Image
Examples
Embodiment 1
[0038] Core preparation
[0039] Tablet core prescription ①: 1000 tablets
[0040] Osalazine Sodium 250g
[0041] Starch 30g
[0042] Sodium carboxymethyl starch 37.5g
[0043] Microcrystalline Cellulose 25g
[0044] 6% PVPk30 Appropriate amount.
[0045] Tablet core prescription ②: 1000 tablets
[0046] Osalazine Sodium 250g
[0047] Modified starch 30g
[0048] Low-substituted hydroxypropyl cellulose 37.5g
[0049] Microcrystalline Cellulose 25g
[0050] 6% PVPk90 Appropriate amount.
[0051] Magnesium Stearate 1.5g.
[0052] Tablet core prescription ③: 1000 tablets
[0053] Osalazine Sodium 250g
[0054] Sodium chloride 30g
[0055] Sodium carboxymethyl starch 37.5g
[0056] Spray dried lactose 25g
[0057] 6% ethyl cellulose appropriate amount
[0059] Tablet core prescription ④: 1000 tablets
[0060] Osalazine Sodium 250g
[0061] Lactose 20g
[0062] Hydroxypropyl Cellulose 37.5g
[0063] Microcrystalline Cellulose 30g
[00...
Embodiment 2
[0083] Preparation of the isolation layer:
[0084] Isolation layer prescription ①: 1000 tablets
[0085] Hypromellose 9.6g
[0086] Macrogol 2000 2.4g
[0087] Diethyl phthalate 1.2g
[0088] 60% ethanol 140g.
[0089] Isolation layer prescription ②: 1000 tablets
[0090] Hypromellose 9.6g
[0091] Macrogol 2000 4.8g
[0092] Diethyl phthalate 1.8g
[0093] 80% ethanol 140g.
[0094] Isolation layer prescription ③: 1000 tablets
[0095] Hypromellose 9.6g
[0096] Macrogol 2000 4.8g
[0097] Triethyl citrate 2.4
[0098] 95% ethanol 140g.
[0099] Isolation layer prescription ④: 1000 tablets
[0100] Hypromellose 9.6g
[0101] Macrogol 2000 4.8g
[0102] Triethyl citrate 1.2
[0103] 95% ethanol 140g.
[0104] The isolation layer prescriptions ①-④ are all coated with high-efficiency coating machines, and the amount of coating solution varies with the amount of tablet cores. Efficient coating machine specific
[0105] The parameters are as follows:
[0106] ...
Embodiment 3
[0109] Colonic coating layer preparation:
[0110] Prescription of colonic coating layer ①: 1000 tablets
[0111] Eudregit S100 20.5g
[0112] Eudregit L100 5g
[0113] Macrogol 2000 4.8g
[0114] Diethyl phthalate 1.8g
[0115] 60% ethanol 500
[0116] Colonic coating layer prescription ②: 1000 tablets
[0117] Eudregit S100 20.5g
[0118] Eudregit L100 7g
[0119] Triethyl citrate 5g
[0120] Macrogol 2000 4.8g
[0121] Diethyl phthalate 1.8g
[0122] 60% ethanol 500
[0123] Colonic coating layer prescription ③: 1000 tablets
[0124] Eudregit S100 20g
[0125] Triethyl citrate 8g
[0126] 80% ethanol 500
[0127] Colonic coating layer prescription ④: 1000 tablets
[0128] Eudregit S100 20g
[0129] Macrogol 2000 2g
[0130] 95% ethanol 415
[0131] The formulations of the colonic coating layer are all coated by a high-efficiency coating machine, and the amount of coating solution varies with the amount of the tablet core. The specific parameters of the hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com