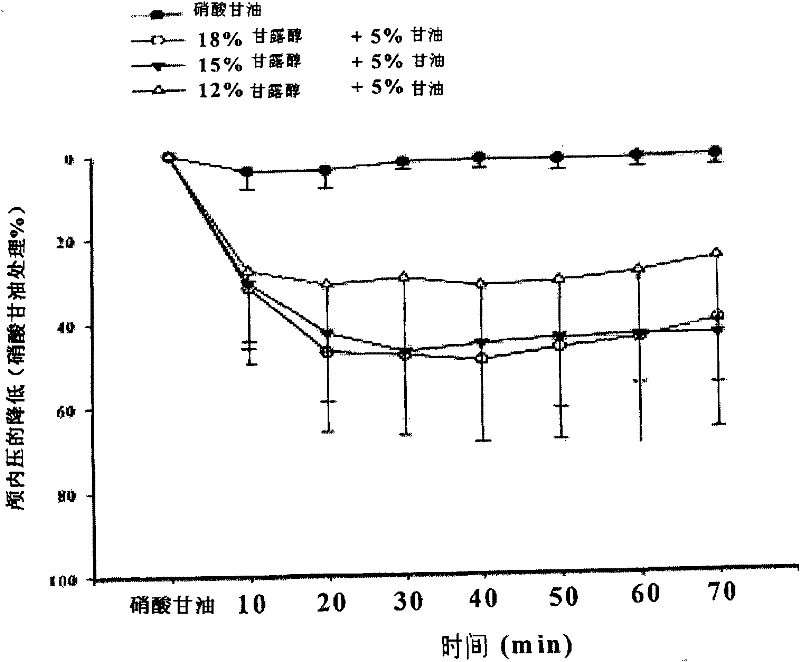
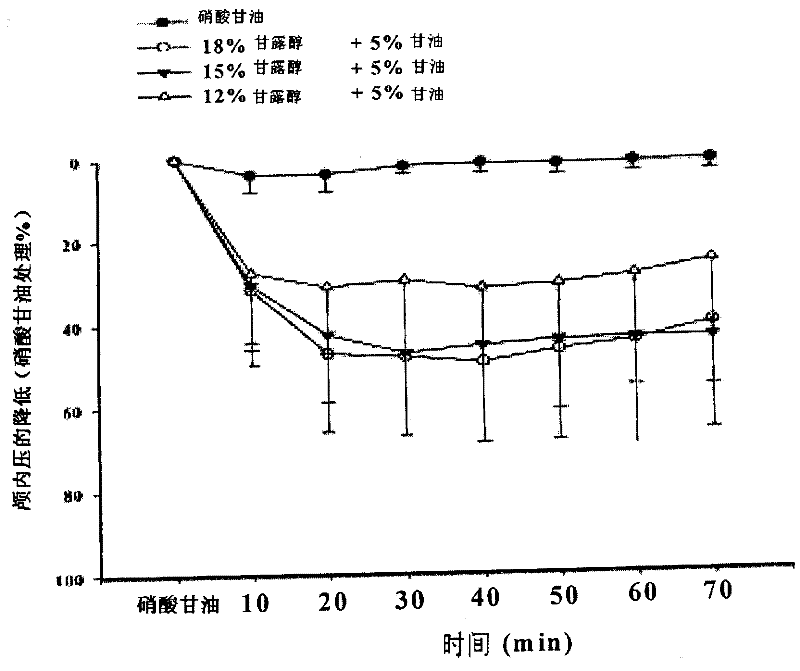
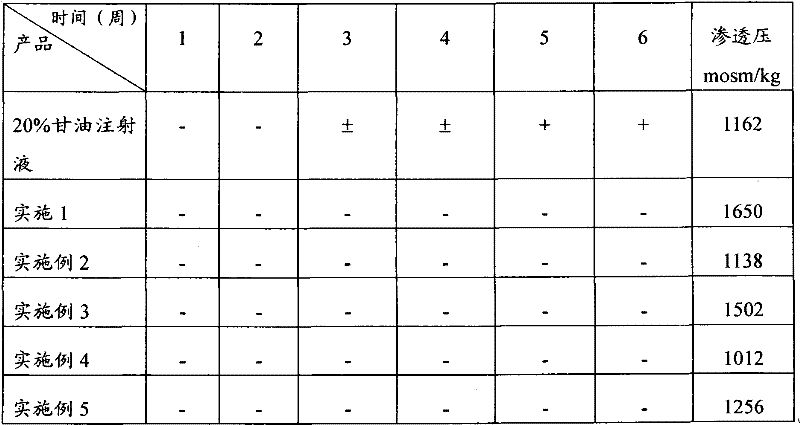
Injection of mannite and glycerol and preparation method thereof
A technology of mannitol and injection, which is applied in the field of mannitol glycerin injection and its preparation, can solve the problems of restricting the clinical use of compound mannitol injection, hidden dangers of drug safety, and unsuitability for clinical first aid, so as to avoid acute renal failure Failure and death, elimination of cerebral edema, mild and slow effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Mannitol glycerin injection, the ratio of each component is as follows:
[0043] Mannitol 18g
[0044] Glycerin 5g
[0045] Add water for injection to 100ml.
[0046] Preparation:
[0047] Dissolve mannitol and glycerin in an appropriate amount of water for injection; add 0.1-0.2% W / V activated carbon for needles, heat to boil, and filter; add the rest of water for injection, mix well, filter, fill and seal, and store at 121°C for 15 Minutes autoclave and ready to serve.
Embodiment 2
[0049] Mannitol glycerin injection, the ratio of each component is as follows:
[0050] Mannitol 15g
[0051] Glycerin 3g
[0052] Add water for injection to 100ml.
[0053] Preparation:
[0054] Dissolve mannitol and glycerin in an appropriate amount of water for injection; add 0.1-0.2% W / V activated carbon for needles, heat to boil, and filter; add the rest of water for injection, mix well, filter, fill and seal, and store at 121°C for 15 Minutes autoclave and ready to serve.
Embodiment 3
[0056] Mannitol glycerin injection, the ratio of each component is as follows:
[0057] Mannitol 15g
[0058] Glycerin 5g
[0059] Add water for injection to 100ml.
[0060] Preparation:
[0061] Dissolve mannitol and glycerin in an appropriate amount of water for injection; add 0.1-0.2% W / V activated carbon for needles, heat to boil, and filter; add the rest of water for injection, mix well, filter, fill and seal, and store at 121°C for 15 Minutes autoclave and ready to serve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com