Electrochemical active material as well as preparation method and application thereof
An active material and electrochemical technology, applied in the field of carbon-free reduction-low temperature synthesis to prepare electrochemical active materials, can solve the problems of large capacity decay rate, long synthesis time, high synthesis temperature, etc., achieve short reaction time, reduce synthesis time, The effect of low synthesis temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of the lithium vanadium phosphate composite material that embodiment 1 is mixed with nanometer Al
[0031] Preparation recipe
[0032]
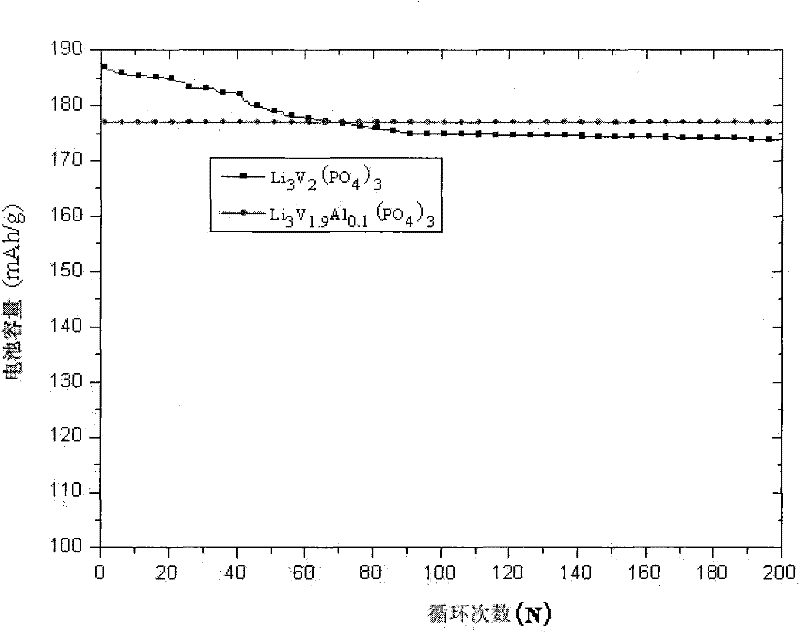
[0033] Lithium dihydrogen phosphate, vanadium pentoxide, vanadium trioxide and nano-Al powder were ball milled at room temperature for 2 hours at a ratio of Li:Al:V:P element molar ratio of 3:0.1:1.9:3, under nitrogen protection conditions The materials are continuously put into the ball milling rotary furnace, the temperature is controlled at 800 degrees, the temperature is kept for 6 hours, and then cooled at room temperature. The product is obtained through milling, testing and packaging.
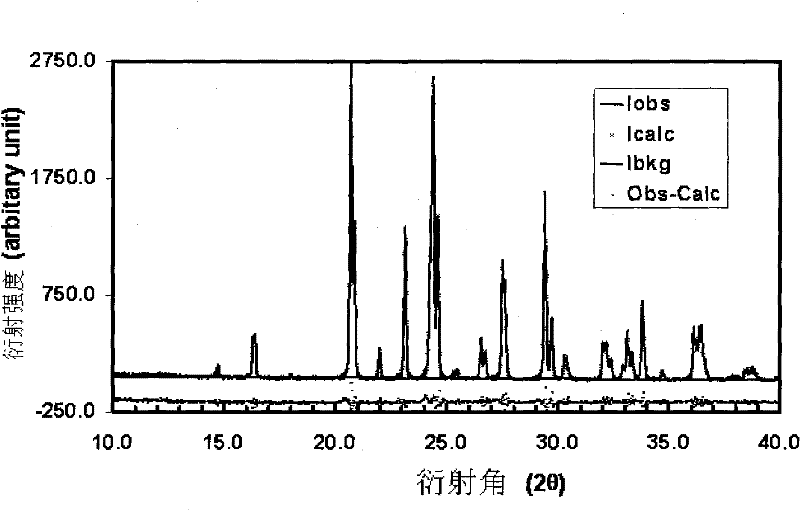
[0034] Sample XRD diffraction analysis: such as image 3 As shown, in the X-ray spectrum, there is no impurity phase, and it is a pure-phase monoclinic lithium vanadium phosphate.
[0035] Electrochemical performance test:
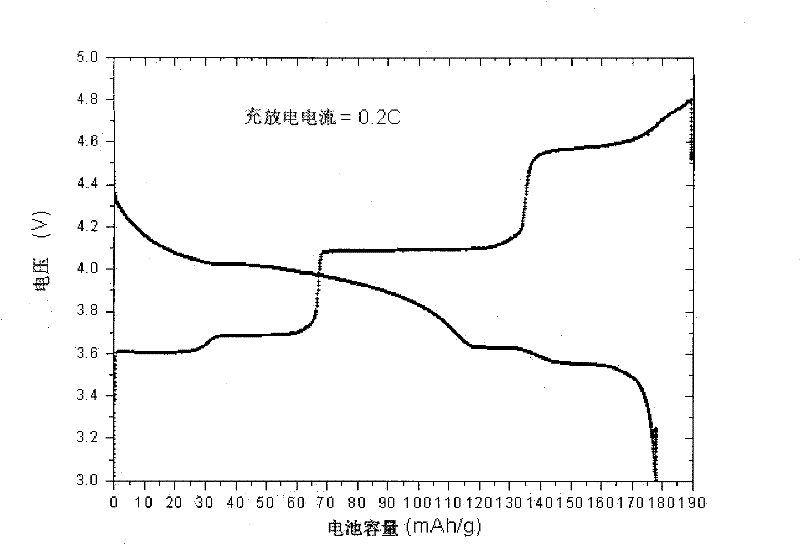
[0036] Add conductive carbon black and binder to the above-prepared lithium vanadium phosphate produ...
Embodiment 2
[0037] The preparation of the lithium vanadium phosphate composite material of embodiment 2 doping nanometer Al
[0038]
[0039]
[0040]Ammonium dihydrogen phosphate, lithium carbonate, vanadium pentoxide, vanadium trioxide and nano-Al powder were ball-milled at room temperature for 6 hours at a ratio of Li:Al:V:P element molar ratio of 3:0.2:1.8:3. Under protected conditions, the materials are continuously fed into the ball milling rotary furnace, the temperature is controlled at 700 degrees, kept for 8 hours, and then cooled at room temperature. The product is obtained through milling, testing and packaging.
[0041] Electrochemical performance test:
[0042] In the electrochemical test process, conductive carbon black and binder are added to make pole pieces, and metal lithium sheets are used as counter electrodes for electrochemical tests. The maximum discharge capacity of the material prepared in this embodiment is 167mAh / g, such as image 3 shown.
Embodiment 3
[0043] The preparation of the lithium vanadium phosphate composite material of embodiment 3 doping nanometer Al
[0044] Preparation recipe
[0045]
[0046] Ammonium dihydrogen phosphate and lithium carbonate, vanadium pentoxide, vanadium trioxide and nano-titanium powder were ball-milled at room temperature for 6 hours at a ratio of Li:Ti:V:P element molar ratio of 3:0.1:1.9:3, Under protected conditions, the materials are continuously fed into the ball milling rotary furnace, the temperature is controlled at 700 degrees, kept for 8 hours, and then cooled at room temperature. The product is obtained through milling, testing and packaging.
[0047] During the battery test process, conductive agents and binders are added, pole pieces are made, and metal lithium sheets are used as the test counter electrode. The tap density of the material prepared in this embodiment is greater than 1.2g / cm 3 , The electrochemical discharge gram capacity of the electrode material is great...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com