High value-added greening comprehensive utilization method for medium and low-grade zinc oxide ores
A technology for comprehensive utilization of zinc oxide ore, applied to chemical instruments and methods, silicon oxide, silicon dioxide, etc., can solve problems such as single product structure, large consumption of sulfuric acid, low zinc content in leachate, etc., and achieve simple process flow, The effect of simple equipment and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
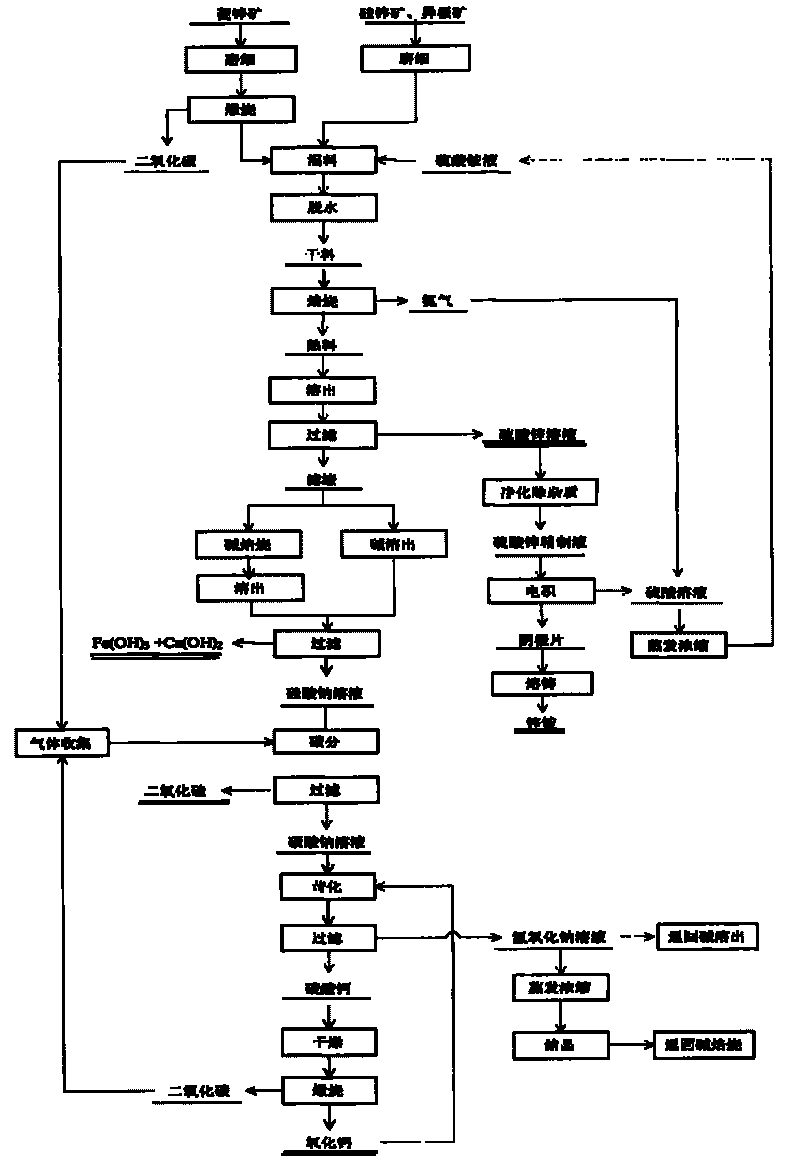
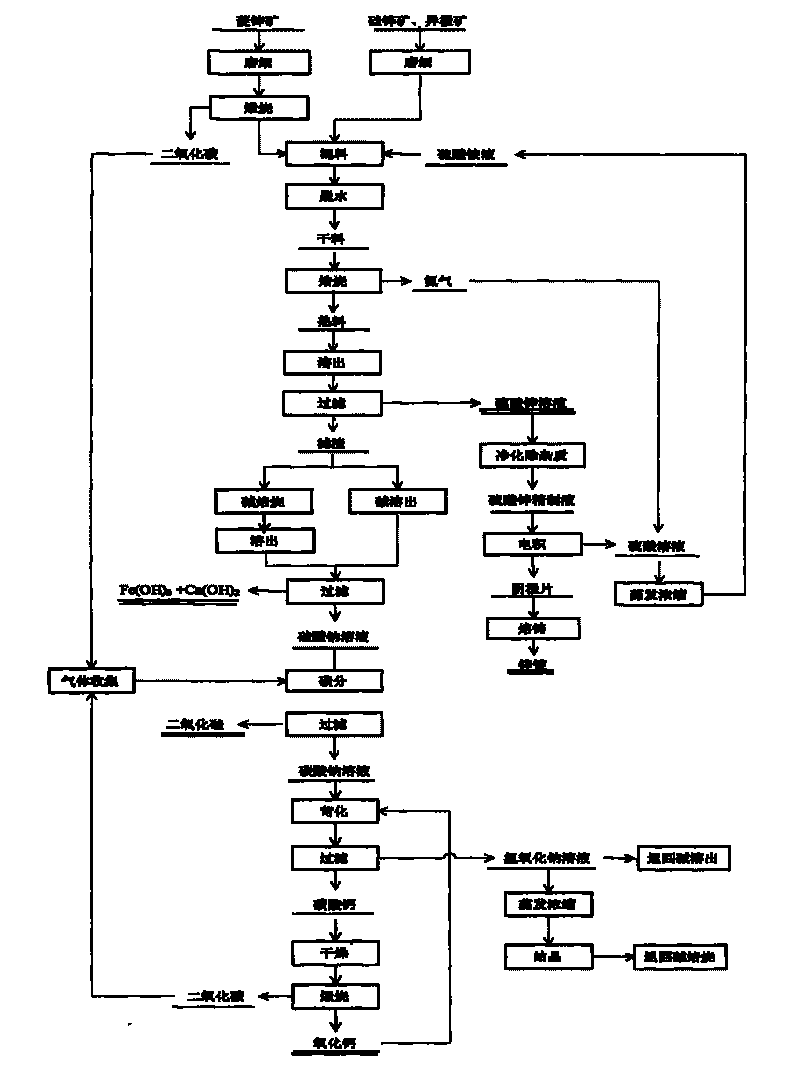
[0045] The composition of zinc oxide ore used is: Zn 28.3%, Fe 2.6%, CaO 1.72%, Pb 3.01%, Al 0.02%, and Cu 0.01%. Zinc exists mainly as smithsonite in minerals.
[0046] The zinc oxide ore is crushed, pulverized to less than 80μm, calcined at 900°C for 3h, and mixed evenly with the ammonium sulfate solution. Among them, the molar ratio of zinc oxide to ammonium sulfate in the ore is 1:3, and the concentration of ammonium sulfate is 40%. Dry and dehydrate for 2 hours below 200°C, then heat up to 500°C for roasting, keep it for 2 hours, and carry out roasting reaction. The roasted product is cooled, dissolved in water, and then subjected to solid-liquid separation. The filtrate is zinc sulfate, iron sulfate and ferrous sulfate, and the filter residue is silica, ferric oxide, etc.
[0047] The filtrate is impurity-removed by the yellow ammonium iron vitriol method to obtain the zinc sulfate refined solution and then electrolyzed. The cathode sheet prepared by the electrowinning wil...
Embodiment 2
[0052] The composition of zinc oxide ore used is: Zn 18.81%, Fe 4.67%, Pb 0.95%, S 2.04%, SiO 2 44.99%, Al 2 O 3 4.16%, CaO 10.54%, MgO 0.48%. Zinc mainly exists in minerals as hemimorphite and willemite.
[0053] The zinc oxide ore is crushed, pulverized to less than 80 μm, and mixed evenly with the ammonium sulfate solution. Among them, the molar ratio of zinc oxide to ammonium sulfate in the ore is 1:4, and the concentration of ammonium sulfate is 38%. Dry and dehydrate for 2 hours below 200°C, then heat up to 400°C for roasting, keep it for 3 hours, and carry out roasting reaction. The roasted product is cooled, dissolved in water, and then subjected to solid-liquid separation. The filtrate is zinc sulfate, iron sulfate and ferrous sulfate, and the filter residue is silica, ferric oxide, and calcium sulfate.
[0054] The filtrate is impurity-removed by the yellow ammonium iron vitriol method to obtain the zinc sulfate refined solution and then electrolyzed. The cathode she...
Embodiment 3
[0059] The composition of zinc oxide ore used is: Zn 11.43%, Fe 16.18%, SiO 2 27.98%, Al 2 O 3 6.18%, CaO 7.52%, MgO 0.17%, Pb 0.68%, Mn 1.76%. Zinc mainly exists as heteropolar ore in minerals.
[0060] The zinc oxide ore is crushed, pulverized to less than 80 μm, and mixed evenly with the ammonium sulfate solution. Among them, the molar ratio of zinc oxide to ammonium sulfate in the ore is 1:6, and the concentration of concentrated ammonium sulfate is 40%. Then it is dried and dehydrated below 150°C for 3 hours, then heated to 450°C for roasting, and kept for 2 hours for roasting reaction. The roasted product is cooled, dissolved in water, and then subjected to solid-liquid separation. The filtrate is zinc sulfate, iron sulfate and ferrous sulfate, and the filter residue is silicon dioxide, ferric oxide and calcium sulfate.
[0061] The filtrate is impurity-removed by the yellow ammonium iron vitriol method to obtain the zinc sulfate refined solution and then electrolyzed. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com