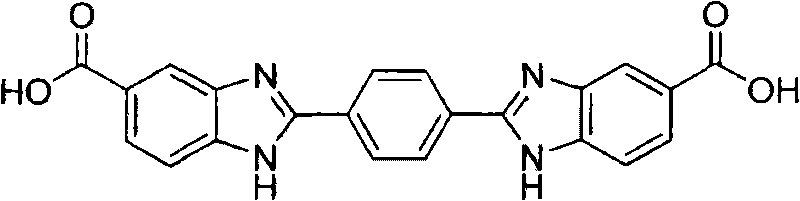
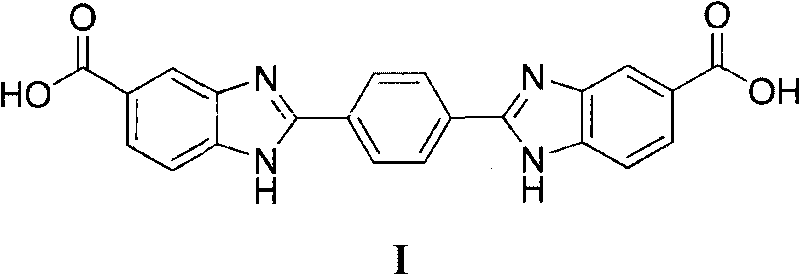
2,2'-(1,4-phenylene)bi(benzimidazole-5-carboxylic acid) and preparation method thereof
A technology of benzimidazole and phenylene, applied in the field of new compounds and their preparation, can solve problems such as difficult separation, and achieve the effect of high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 The preparation of 2,2'-(1,4-phenylene)bis(benzimidazole-5-carboxylic acid)
[0043] 12.4mmol 3,4-diaminobenzoic acid, 10mL dimethyl sulfoxide, heated in an oil bath, raised the temperature to 80°C, added dropwise 6.2mmol terephthalaldehyde in dimethyl sulfoxide solution, stirred for 2.0h, and added to the reaction system Add 4.2mmol of 3-nitro-4-aminobenzoic acid, continue stirring for 2.0h; pour the reaction liquid into 80mL of ethanol, let it stand, filter, and dry to obtain a light brown solid with a yield of 94.6%, mp.≥300°C.1 HNMR (400MHz, DMSO-d 6 ), δ: 7.84~8.29(m, 6H, 2×C 6 h 3 ), 8.41(s, 4H, C 6 h 4 ), 12.78(s, 2H, 2×NH), 13.40(s, 2H, 2×CO 2 H); IR (KBr, cm -1 )υ: 3367 (broad peak, O-H, N-H), 3212 (Ph-H), 1677, 1624 (C=O, C=N), 1543 (Ph), 1322, 1306, 1016, 963 (Ph). Recover 3,4-diaminobenzoic acid as a raw material.
Embodiment 2
[0044] Example 2 Preparation of 2,2'-(1,4-phenylene)bis(benzimidazole-5-carboxylic acid)
[0045] 12.4mmol 3,4-diaminobenzoic acid, 10mL acetonitrile, heated in an oil bath, raised the temperature to 80°C, added dropwise 6.2mmol terephthalaldehyde acetonitrile solution, stirred for 1.5h, added 4.2mmol 3-amino- 4-Nitrobenzoic acid, continue to stir and react for 2.0 h; the reaction solution is poured into 80 mL of ethanol, allowed to stand, filtered, and dried to obtain a light brown solid with a yield of 94.3%, mp.≥300°C. 1 H NMR (400MHz, DMSO-d 6 ), δ: 7.84~8.29(m, 6H, 2×C 6 h 3 ), 8.41(s, 4H, C 6 h 4 ), 12.78(s, 2H, 2×NH), 13.40(s, 2H, 2×CO 2 H); IR (KBr, cm -1 )υ: 3367 (broad peak, O-H, N-H), 3212 (Ph-H), 1677, 1624 (C=O, C=N), 1543 (Ph), 1322, 1306, 1016, 963 (Ph). Recover 3,4-diaminobenzoic acid as a raw material.
Embodiment 3
[0046] Example 3 Preparation of 2,2'-(1,4-phenylene)bis(benzimidazole-5-carboxylic acid) (cerium ammonium nitrate-hydrogen peroxide composite oxidation method)
[0047] 12.4mmol 3,4-diaminobenzoic acid, 10mL N,N-dimethylformamide, heated in an oil bath, raised to 80°C, added dropwise 6.2mmol terephthalaldehyde N,N-dimethylformamide solution, Stir the reaction for 1 hour, cool down to 60°C, add 0.67g of cerium ammonium nitrate and 5mL of 30% hydrogen peroxide to the reaction system, and continue to stir and react for 20 minutes; the reaction solution is poured into 80mL of ethanol, left to stand, filtered, and dried to obtain 2.34g of light brown Solid, yield 94.7%, mp.≥300°C. 1 H NMR (400MHz, DMSO-d 6 ), δ: 7.84~8.29(m, 6H, 2×C 6 h 3 ), 8.41(s, 4H, C 6 h 4 ), 12.78(s, 2H, 2×NH), 13.40(s, 2H, 2×CO 2 H); IR (KBr, cm -1 )υ: 3367 (broad peak, O-H, N-H), 3212 (Ph-H), 1677, 1624 (C=O, C=N), 1543 (Ph), 1322, 1306, 1016, 963 (Ph).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com