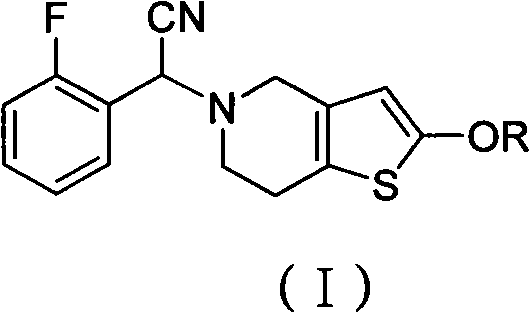
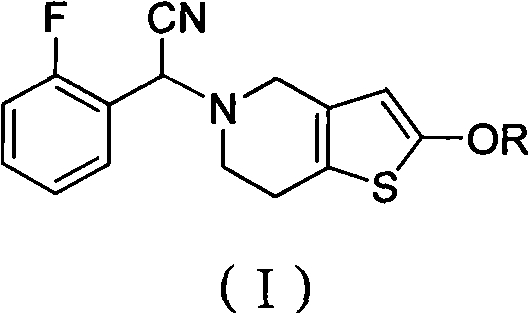
Prasugrel intermediate and preparation method thereof
An intermediate and reaction time technology, applied in the field of prasugrel intermediate and its preparation, can solve the problems of low yield, high environmental protection pressure, high cost, etc., and achieve simple process operation, cheap and easy-to-obtain raw materials, and low environmental protection pressure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 Cyanide reaction
[0055]Dissolve 189g of o-fluorobenzyl bromide (1mol) in a solution composed of 500mL of toluene and 500mL of water, add 58.8g of sodium cyanide (1.2mol), stir and dissolve, then add 7.4g of tetrabutylammonium bromide (0.02mol ) to form a reaction system, the reaction system was heated to 55 ° C and stirred for 10 hours. Thin layer chromatography (TLC) showed that the raw materials were completely reacted. After rectification under reduced pressure, 115 g of white liquid was obtained with a yield of 85%.
[0056] White liquid: 1H NMR (δ, CDCl3): 3.78(s, 2H), 7.10~7.19(m, 1H), 7.21~7.23(m, 1H), 7.34~7.37(m, 1H), 7.45~7.49(m , 1H); MS (75eV), m / z (%): 136 (M + +1, 100).
[0057] The above results show that the prepared white liquid is o-fluorophenylacetonitrile, ie 2-(2-fluorophenyl)acetonitrile.
Embodiment 2
[0058] Example 2 bromination reaction
[0059] Dissolve 13.5g of o-fluorophenylacetonitrile (0.1mol) in 100mL of dichloromethane to form a reaction system, lower the temperature of the reaction system to 0°C, and slowly add 50mL of bromine (17.6g, 0.11mol ) in dichloromethane solution, the temperature of the reaction system was raised to room temperature after dripping, and after stirring for 3 hours, TLC detected that the raw material had reacted completely, and the saturated solution of 200mL sodium sulfite was added to the reaction system, and the organic phase was dried after standing for stratification. After concentrating, about 17.3 g of the brominated product was obtained as a colorless liquid with a yield of 81%.
[0060] Brominated products: 1 H NMR (δ, CDCl3): 5.76(s, 3H), 7.14~7.18(m, 1H), 7.28~7.31(m, 1H), 7.07(s, 1H), 7.46~7.48(m, 1H), 7.71 ~7.75(m, 1H); MS(75eV), m / z(%): 215(M + +1, 100).
[0061] The above results show that the prepared brominated product i...
Embodiment 3
[0062] Example 3 bromination reaction
[0063] Dissolve 13.5g of o-fluorophenylacetonitrile (0.1mol) in 100mL of dichloromethane to form a reaction system, lower the temperature of the reaction system to 5°C, and slowly add 50mL of N-bromosuccinyl The dichloromethane solution of amine (19.6g, 0.11mol), after dropping, the temperature of the reaction system was raised to room temperature, and after stirring for 3 hours, TLC detected that the raw material had reacted completely, and the saturated solution of 200mL of sodium sulfite was added to the reaction system, and allowed to stand After separation, the organic phase was dried and concentrated to obtain 18.8 g of brominated product with a yield of 88%.
[0064] Brominated product: 1H NMR (δ, CDCl3): 2.30(s, 3H, CH3), 2.39(s, 3H, CH3), 6.87~6.89(m, 1H), 7.07(s, 1H), 7.41~7.43( m, 1H); MS (75eV), m / z (%): 184 (M+, 61), 186 (60), 105 (100), 103 (20), 79 (16), 77 (21).
[0065] The above results show that the prepared brominat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap