Preparation method of acanthopanax effervescent tablet and products thereof
A kind of Acanthopanax and effervescent tablet technology, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of poor stability of preparations, poor foaming effect, and easy sticking, etc. problem, to achieve the effect of reducing moisture absorption, strong operability and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
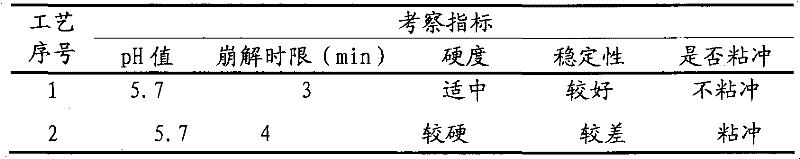
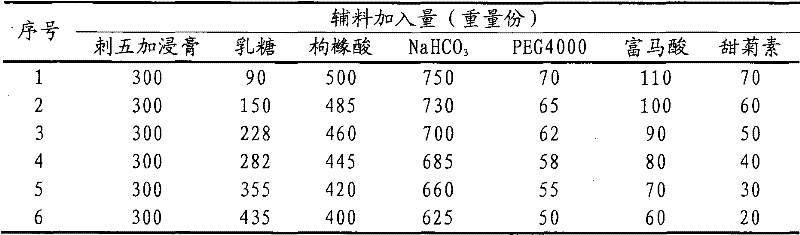
[0040] Weigh 300g of Acanthopanax extractum, 150g of lactose, 485g of citric acid, 730g of sodium bicarbonate, 65g of polyethylene glycol 4000, 100g of fumaric acid, and 60g of cyclamate;
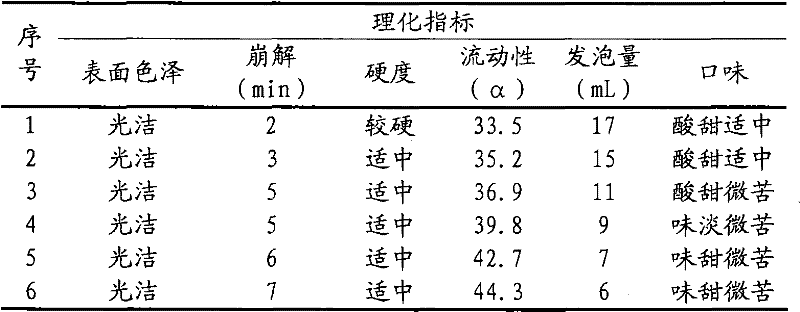
[0041] According to the process described in Test Example 1, the Acanthopanax saponiosa extract and lactose were mixed evenly, dried, pulverized into fine powder, and set aside; measure 500ml of ethanol solution to dissolve polyethylene glycol 4000, and add sodium bicarbonate to polyethylene glycol Wrap it in the solution to obtain a mixed solution containing inclusions, spray it into a rotating coating pan filled with Acanthopanax extractum and lactose mixed fine powder, sieve, and granulate to obtain granules for later use; Sieve citric acid and cyclamate to make granules, mix with the above granules and fumaric acid fine powder, and press 1000 tablets (1.8g / tablet). All the inspection indexes of the prepared granules are in compliance with the regulations, and the accelerated test has be...
Embodiment 2
[0043] Weigh 300g of Acanthopanax extirgae extract, 175g of lactose, 370g of citric acid, 555g of sodium bicarbonate, 60g of polyethylene glycol 4000, 80g of fumaric acid, and 50g of cyclamate;
[0044] According to the process described in Test Example 1, mix Acanthopanax saponiosa extract and lactose evenly, dry, pulverize into fine powder, and set aside; measure 400ml of ethanol solution to dissolve polyethylene glycol 4000, and add sodium bicarbonate to the polyethylene glycol solution Wrap it up to obtain a mixed solution containing inclusions, spray it in a rotary coating pan filled with Acanthopanax extractum and lactose mixed fine powder, sieve, and granulate to obtain granules for later use; Citric acid and cyclamate were sieved to make granules, mixed with the above granules and fumaric acid fine powder, and pressed into 1000 tablets (1.5g / tablet). All the inspection indexes of the prepared granules are in compliance with the regulations, and the accelerated test has...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com