Efficient electrochemical reactor of electro-catalysis in-situ hydrogen peroxide
A hydrogen peroxide, in-situ generation technology, applied in the electrolysis process, electrolysis components, cells, etc., can solve the problems of rare reactor detailed reports, achieve the effects of improving safety, simplifying assembly, and increasing utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
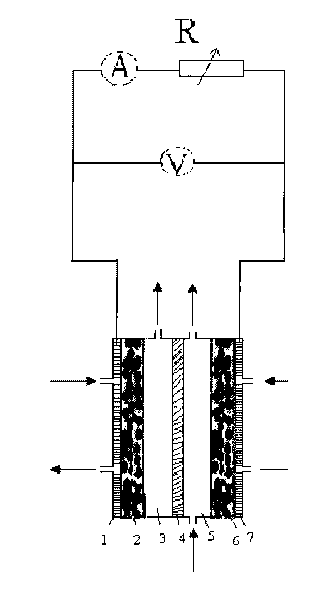
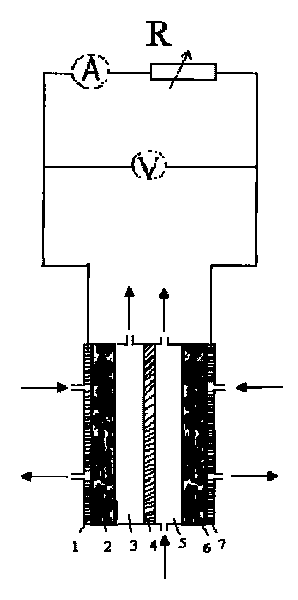
[0025] An anode gas diffusion electrode with a platinum loading of 0.5 mg / cm2 and a partially graphitized XC-72 gas diffusion electrode were prepared. Put the anode gas electrode, Nafion117 membrane and cathode diffusion electrode into the battery respectively. Configure 2Mol / L NaOH solution, and inject the electrolyte solution into the cathode and anode reaction chambers respectively. Cathode and anode respectively pass through O 2 、H 2 , the flow rate is 80mL / min, the temperature of the battery is controlled at about 0 degrees, and it is discharged in the form of a short circuit, such as figure 1 , after two hours, the concentration of hydrogen peroxide obtained on the cathode side is 2%, and the maximum current density is 50mA / cm 2 .
Embodiment 2
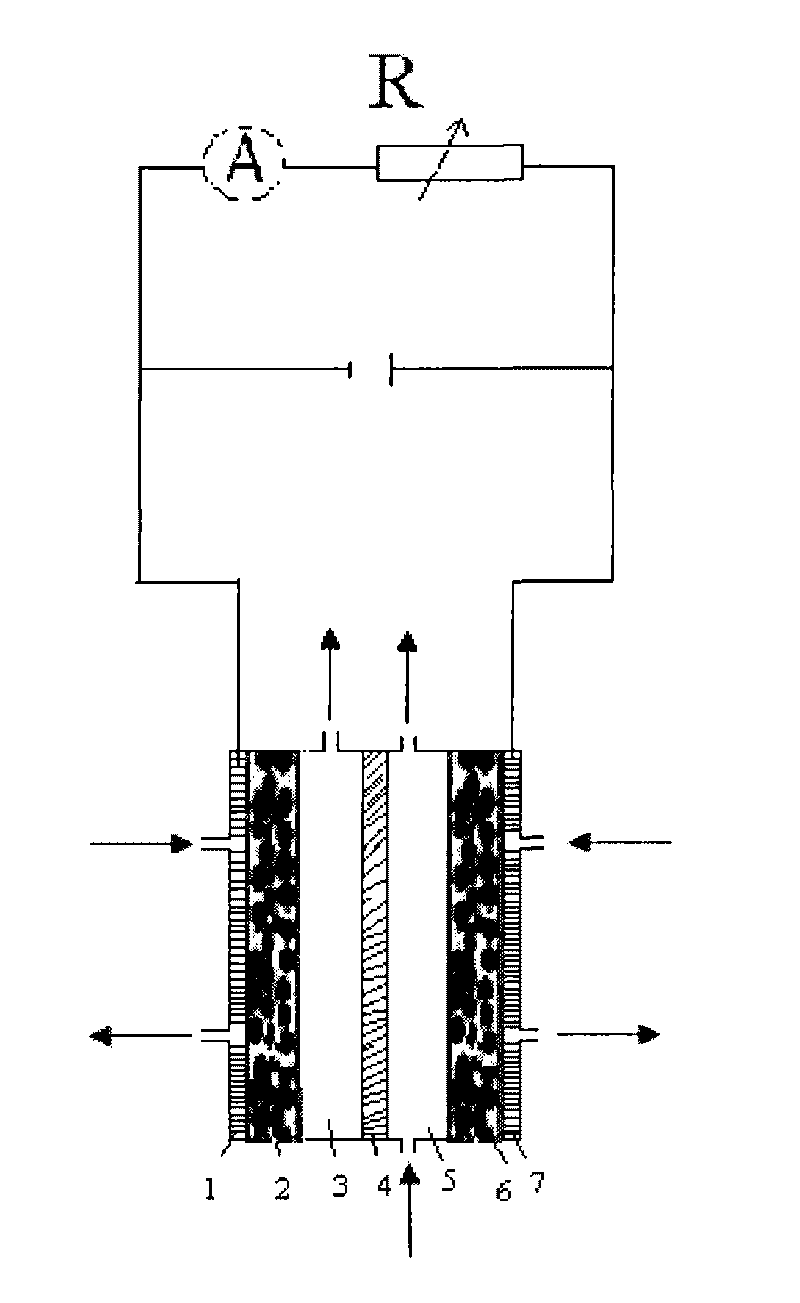
[0027] Prepare a platinum loading of 0.5 mg / cm 2The anode gas diffusion electrode and the partially graphitized XC-72 gas diffusion electrode. Put the anode gas electrode, Nafion117 membrane and cathode diffusion electrode into the battery respectively. Configure 2Mol / L NaOH solution, and inject the electrolyte solution into the cathode and anode reaction chambers respectively. Cathode and anode respectively pass through O 2 、H 2 , the flow rate is 80mL / min, and the temperature of the battery is controlled at about 0 degrees. Apply a voltage of 1V to both ends of the reactor through an external circuit, such as figure 2 , after two hours, the concentration of hydrogen peroxide on the cathode side is 4%, and the maximum current density is 100mA / cm 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com