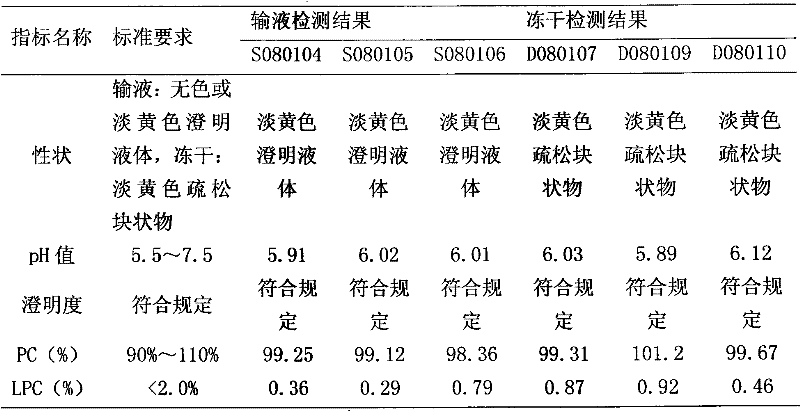
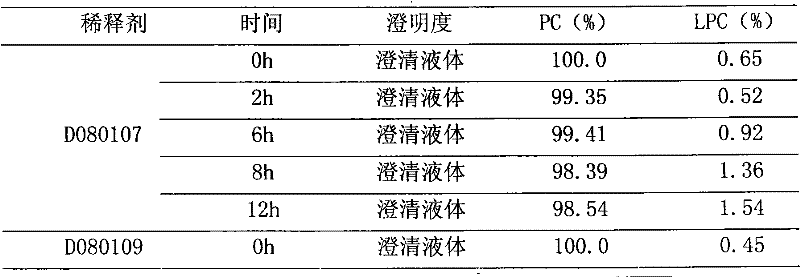
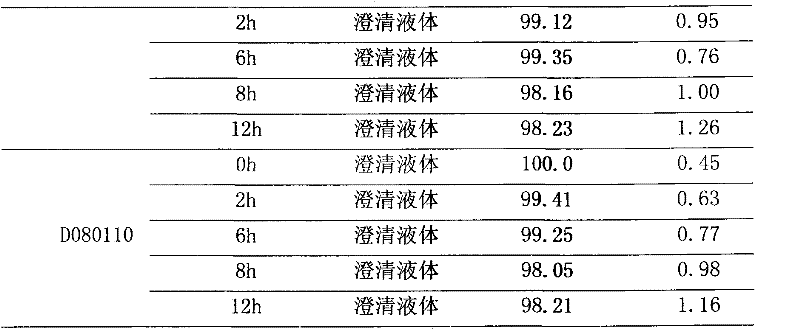
Polyene phosphatidyl choline intravenous preparation and preparation method thereof
A polyene phosphatidylcholine and preparation technology, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of drug liquid pollution, high blood sugar side effects, and unseen content reports and other problems, to achieve the effect of convenient clinical use, safe clinical application and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 (specification 100ml: 232.5mg)
[0020] Put 250 g of polyene phosphatidylcholine and 500 g of sodium cholate in a high-speed tissue masher, add 60,000 ml of water for injection, and stir under nitrogen flow until a uniform translucent colloidal dispersion system is formed. Add activated carbon for adsorption for 15 minutes, and use a No. 3 vertical fusion filter for coarse filtration under nitrogen flow. Add 5000g of xylitol and 20000ml of water for injection, circulate and homogenize several times, add water to a sufficient amount of 100000ml, circulate and homogenize evenly. After passing through a 0.22 μm microporous membrane filter (sampling inspection), filling, filling with nitrogen, fusion sealing, sterilizing at 121° C. for 15 minutes. Inspection, packaging.
Embodiment 2
[0021] Embodiment 2 (specification 250ml: 465.0mg)
[0022] Put 250 g of polyene phosphatidylcholine and 350 g of sodium cholate in a high-speed tissue masher, add 150,000 ml of water for injection, and stir under nitrogen flow until a uniform translucent colloidal dispersion system is formed. Add activated carbon for adsorption for 15 minutes, and use a No. 3 vertical fusion filter for coarse filtration under nitrogen flow. Add 12500g of xylitol and 50000ml of water for injection, circulate and homogenize several times, add water to a sufficient amount of 250000ml, circulate and homogenize evenly. After passing through a 0.22 μm microporous membrane filter (sampling inspection), filling, filling with nitrogen, fusion sealing, sterilizing at 121° C. for 15 minutes. Inspection, packaging.
Embodiment 3
[0023] Embodiment 3 (specification 500ml: 1162.5mg)
[0024] Put 250 g of polyene phosphatidylcholine and 250 g of sodium cholate in a high-speed tissue masher, add 300,000 ml of water for injection, and stir under nitrogen flow until a uniform translucent colloidal dispersion system is formed. Add activated carbon for adsorption for 15 minutes, and use a No. 3 vertical fusion filter for coarse filtration under nitrogen flow. Add 25,000g of xylitol and 125,000ml of water for injection, circulate and homogenize several times, add water to a sufficient amount of 500,000ml, and circulate and homogenize evenly. After passing through a 0.22 μm microporous membrane filter (sampling inspection), filling, filling with nitrogen, fusion sealing, sterilizing at 121° C. for 15 minutes. Inspection, packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com