Method for preparing industrial grade and food grade phosphoric acid by decomposing low grade phosphate rocks with hydrochloric acid
A low-grade, industrial-grade technology, applied in chemical instruments and methods, phosphoric acid, phosphorus compounds, etc., can solve problems such as increased energy consumption, decreased extraction rate, and difficult filtration, to achieve reduced energy consumption and cost, mild extraction conditions, The effect of high extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
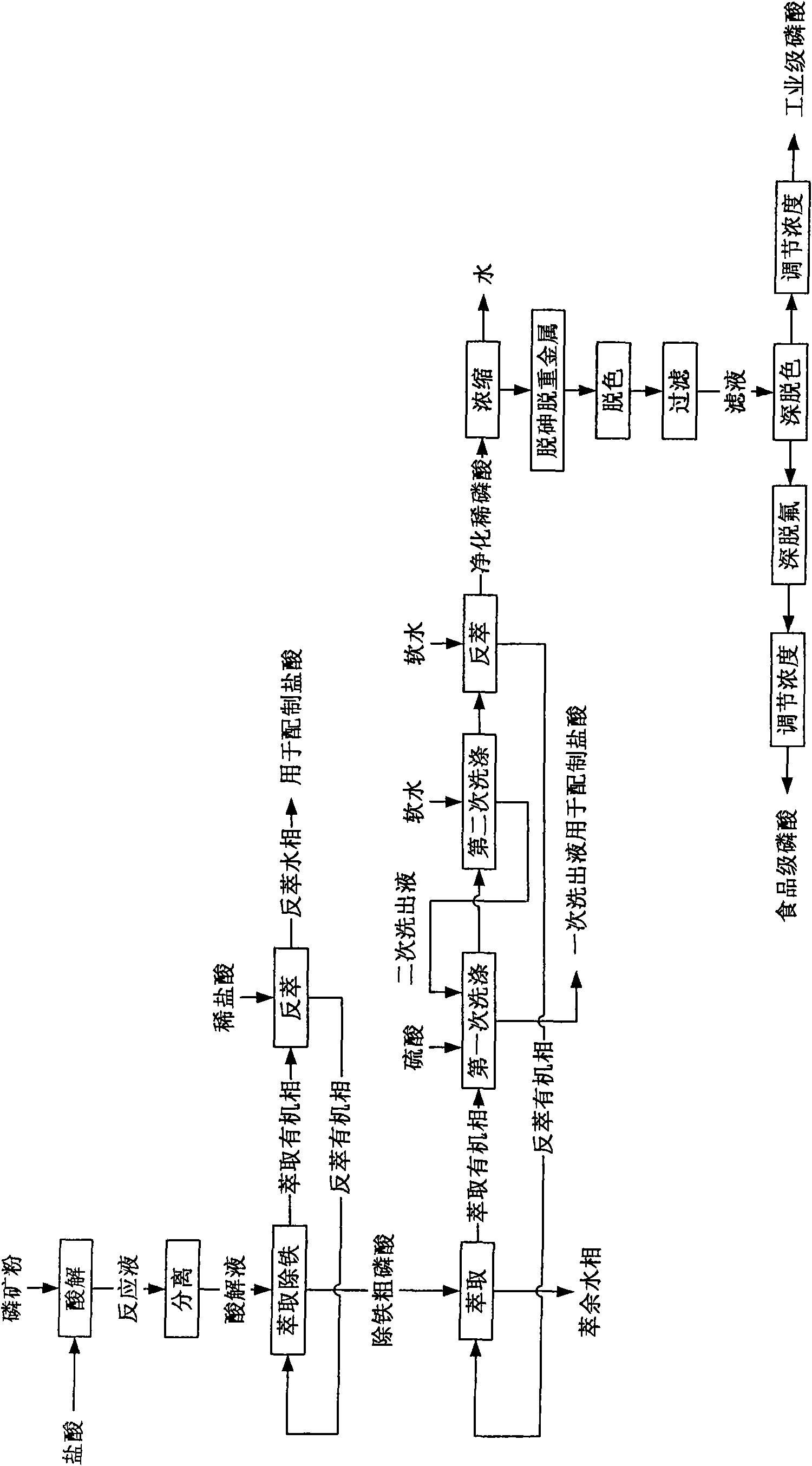
[0045] In this example, medium and low-grade phosphate rock is used to prepare industrial-grade phosphoric acid and food-grade phosphoric acid. For the process steps, see figure 1 , in order as follows:
[0046] (1) Hydrochloric acid acid hydrolysis of phosphate rock powder
[0047] The acid hydrolysis of phosphate rock powder is carried out in an acid hydrolysis tank, the mass concentration of hydrochloric acid is 21%, and the dosage of hydrochloric acid is: 100% of the theoretical amount of hydrochloric acid required when CaO in phosphate rock powder reacts completely with hydrochloric acid; first, add hydrochloric acid to acid hydrolysis Then add phosphate rock powder into hydrochloric acid, react under stirring at room temperature (25°C) and normal pressure, the reaction time is 45 minutes, after the reaction, add polyacrylamide with a molecular weight of 3 million Dalton The polyacrylamide aqueous solution with a mass concentration of 0.15% is flocculated and precipitate...
Embodiment 2
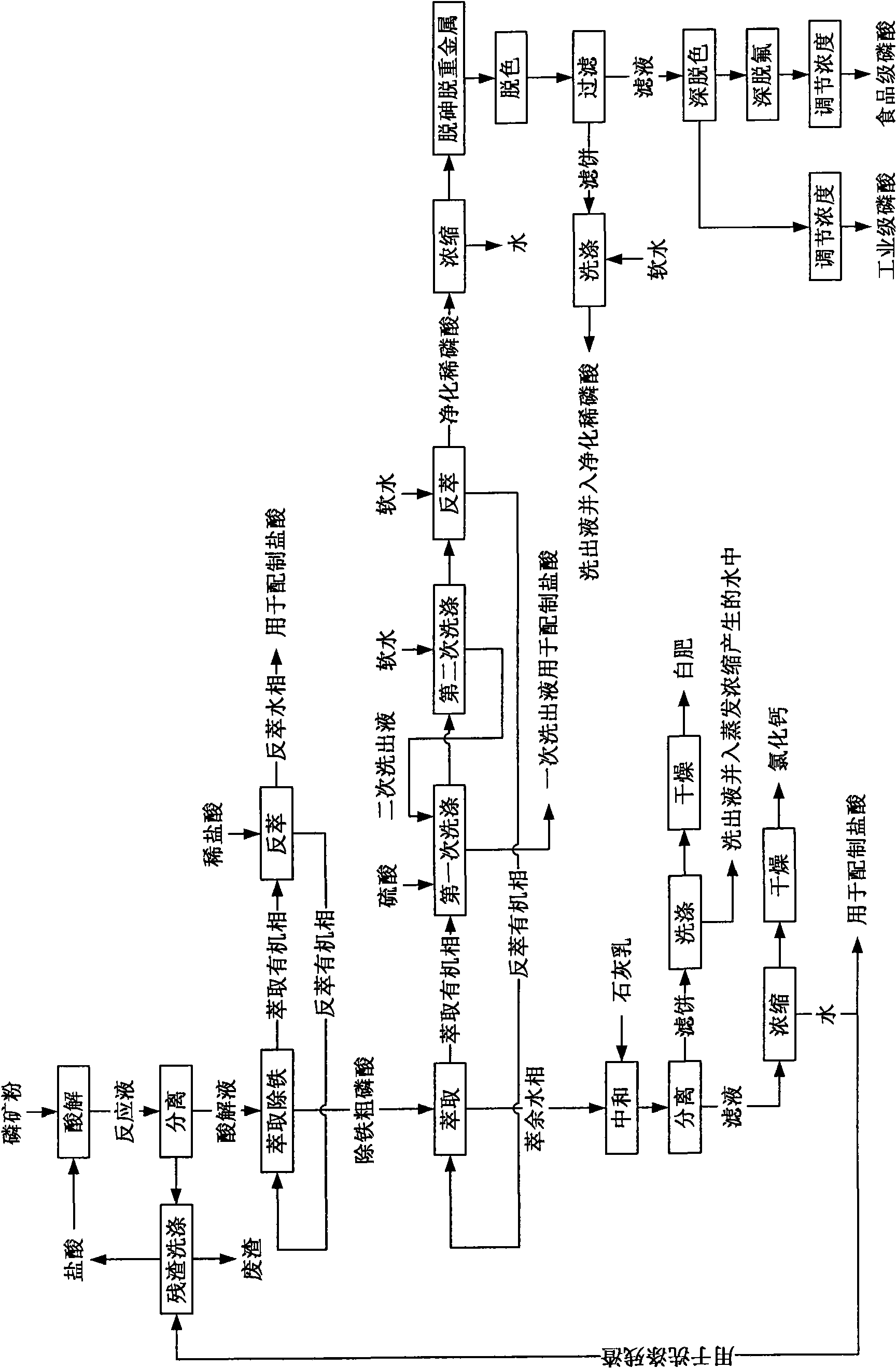
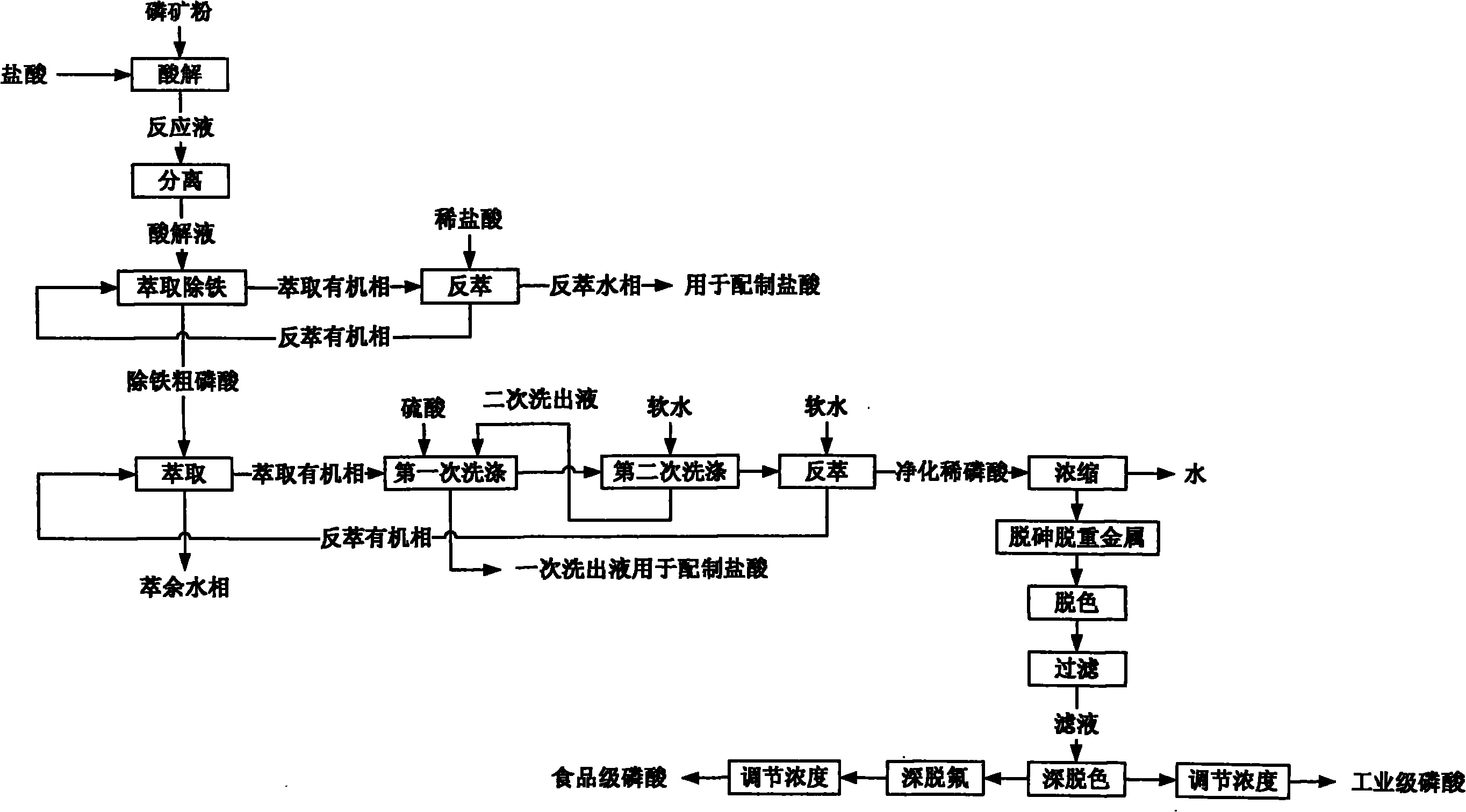
[0070] In this example, industrial-grade phosphoric acid is prepared from medium and low-grade phosphate rock. For the process steps, see figure 2 , in order as follows:
[0071] (1) Hydrochloric acid acid hydrolysis of phosphate rock powder
[0072] The acid hydrolysis of phosphate rock powder is carried out in an acid hydrolysis tank, the mass concentration of hydrochloric acid is 15%, and the dosage of hydrochloric acid is: 105% of the theoretical amount of hydrochloric acid required when CaO in phosphate rock powder reacts completely with hydrochloric acid; first, hydrochloric acid is added to acid hydrolysis Then add phosphate rock powder into hydrochloric acid, react under stirring at room temperature (20°C) and normal pressure, the reaction time is 30 minutes, after the reaction, add polyacrylamide with molecular weight of 3 million Daltons The polyacrylamide aqueous solution with a mass concentration of 0.05% is flocculated and precipitated, and the addition amount o...
Embodiment 3
[0092] In this example, food-grade phosphoric acid is prepared with medium and low-grade phosphate rock. For the process steps, see figure 2 , in order as follows:
[0093] (1) Hydrochloric acid acid hydrolysis of phosphate rock powder
[0094] The acid hydrolysis of phosphate rock powder is carried out in an acid hydrolysis tank, the mass concentration of hydrochloric acid is 26%, and the dosage of hydrochloric acid is: 95% of the theoretical amount of hydrochloric acid required when the CaO in the phosphate rock powder reacts completely with hydrochloric acid; Then add phosphate rock powder into hydrochloric acid, react under stirring at room temperature (28°C) and normal pressure, the reaction time is 60 minutes, after the reaction, add polyacrylamide with molecular weight of 3 million Daltons The polyacrylamide aqueous solution with a mass concentration of 0.3% is flocculated and precipitated, and the addition amount of the polyacrylamide solution is 0.2% of the volume o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com