New method for producing propiconazole
The technology of a propiconazole and a new method is applied in a new field of production of the original propiconazole, can solve the problems of difficult recycling of mixed solvents, low bromination conversion rate, difficult nitration operation, etc., and achieves reduction of solvent treatment costs, The effect of shortening the reaction period and high bromide conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
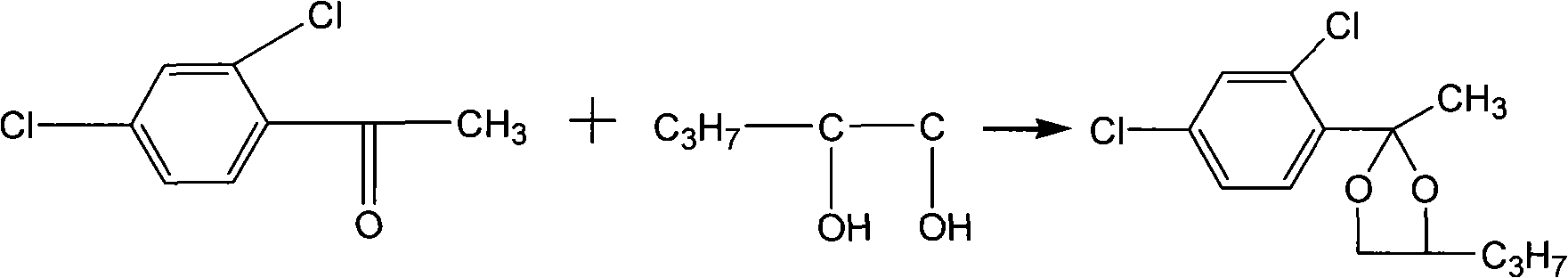
[0027] A new method for the production of the original drug of propiconazole, according to the ratio of the amount of substances 2,4-dichloroacetophenone: 1,2-pentanediol is a ratio of 1: 1.2, 2,4 dichloroacetophenone Ketone and 1,2-pentanediol undergo cyclization reaction under reflux, at this time the temperature is about 86°C, and the reaction time is about 10 hours. Then, after the cyclization reaction is completed, bromination reaction is carried out. First add bromine accounting for 1% to 10% of the total mass of bromine required for the reaction into the reaction system for bromination induction reaction. After 5 to 50 minutes of reaction, white smoke is generated in the kettle, which proves that the initiation is successful, and a large amount of bromine is started. Add the remaining bromine dropwise, and continue the reaction for 0.5 to 3.0 hours for bromination to generate bromide——2-bromomethyl-(2,4-dichlorophenyl)-4-propyl-1,3-dioxol alkyl. In the present inventio...
Embodiment 2
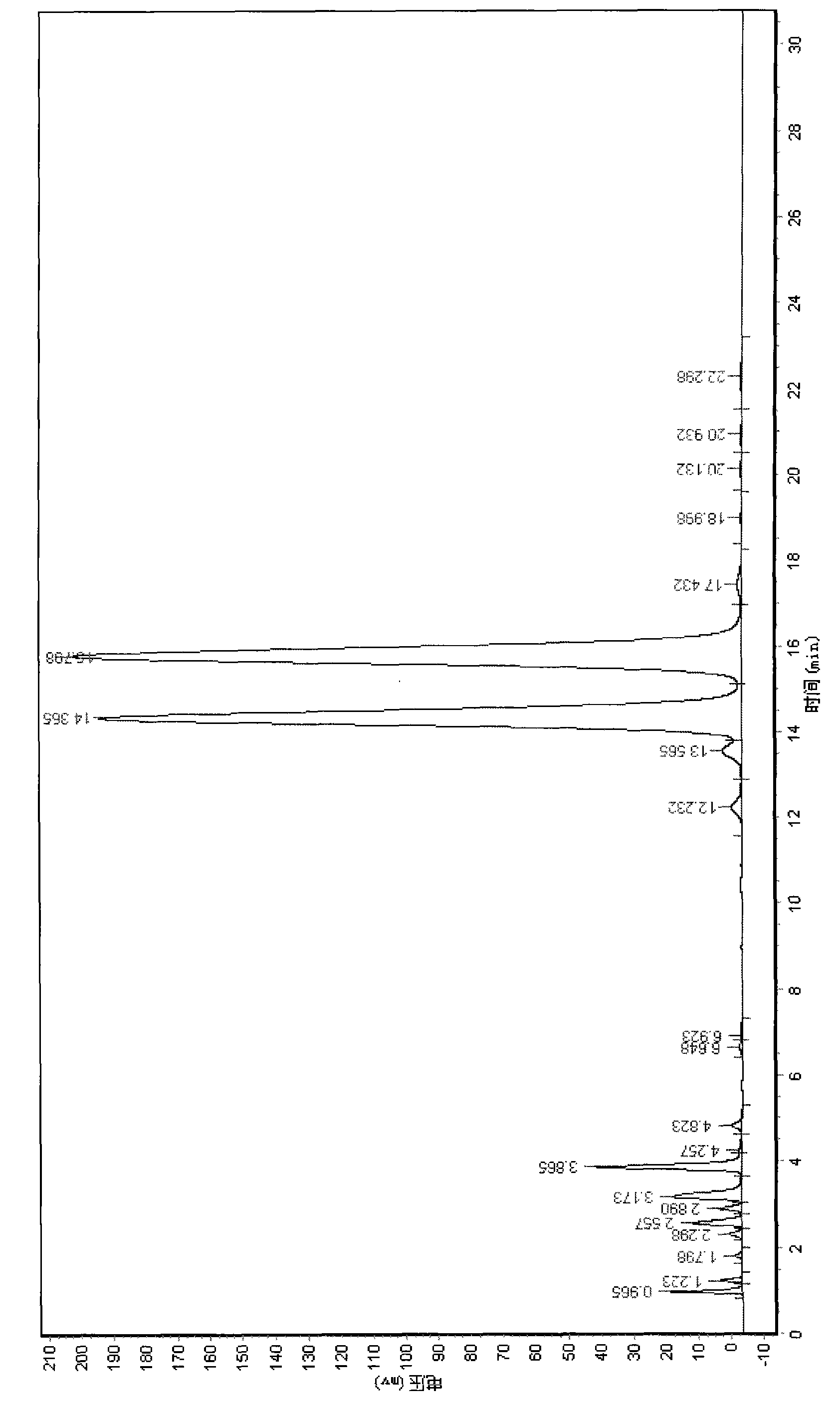
[0030] Add 210KG of 2,4-dichloroacetophenone to the reaction kettle, and react with 145KG of 1,2-pentanediol under the conditions of solvent cyclohexane 1800L and catalyst p-toluenesulfonic acid 10-20Kg, and continuously Separating the water to obtain a cyclohexane solution containing the cyclized product, when 2,4-dichloroacetophenone≤1.0% therein (liquid chromatography, L=150mm-250mm, T=40°C, wavelength 205nm~220nm , the mobile phase is a certain proportion of methanol and water mixed solvent), the temperature is lowered to 20-80° C., and the excess 1,2-pentanediol is separated by standing. After raising the temperature to 40°C, add 1% to 10% of the total amount of bromine required, that is, 2.1 to 21Kg, into the reaction kettle, and keep it warm for about 5 to 50 minutes at 20 to 80°C. White smoke is emitted from the kettle. Accurate, it proves that the bromination initiation is successful. At this time, the remaining bromine is added dropwise at 10-45°C, and the time is co...
Embodiment 3
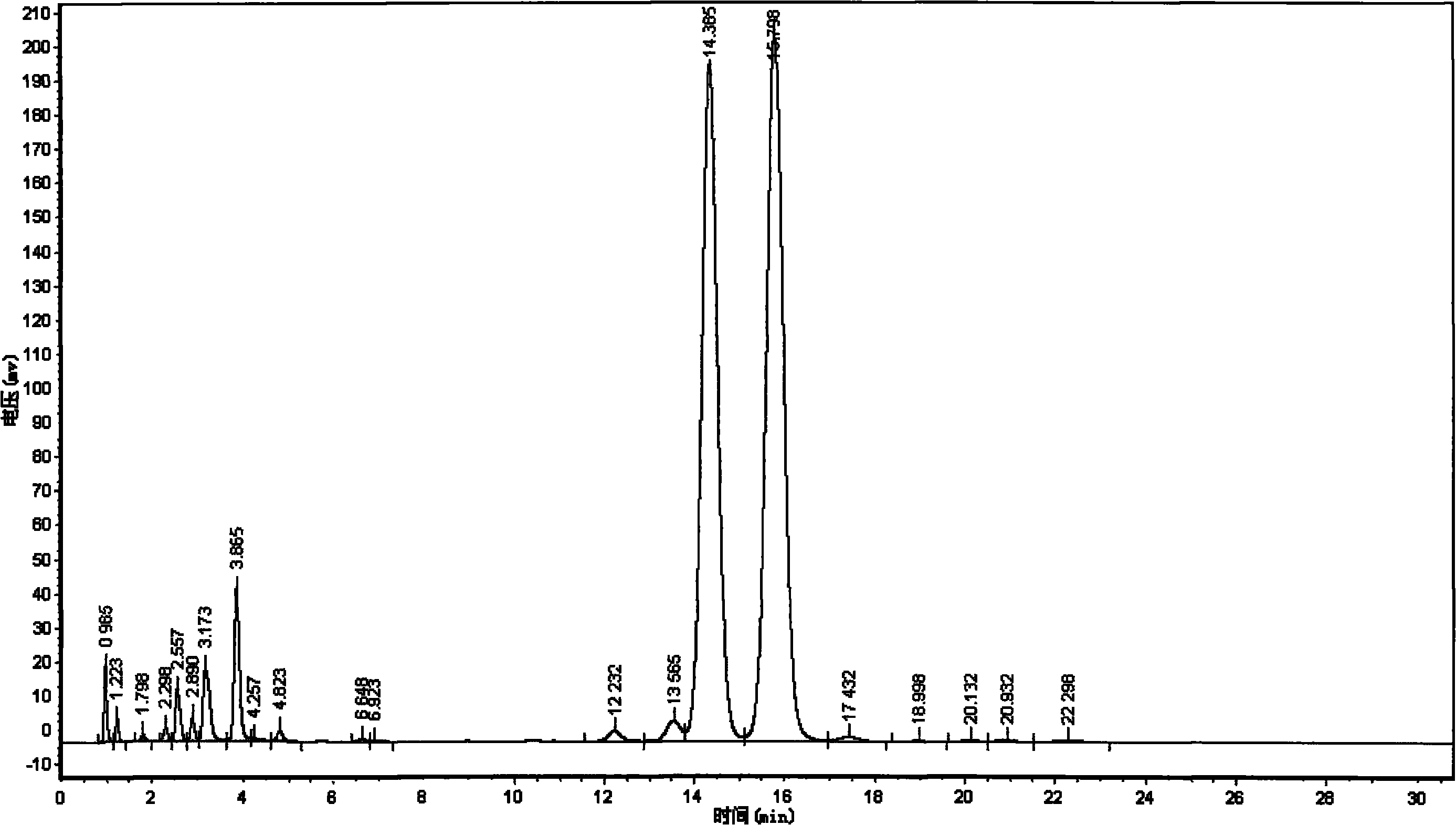
[0045] Add 105KG (95%wt) of 1,2,4-triazole and 97KG (92%wt) of potassium hydroxide to the reaction kettle, under the condition of 600L pyrrolidone as solvent, 140~200℃ with intermittent slight negative pressure (- 0.02-0.08MPa) backflow to separate water, the reaction time is 4-5 hours, and the water content is controlled below 2.8%wt (measured by a micromoisture analyzer). To obtain 1,2,4-triazole potassium, add bromide 2-bromomethyl-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolane prepared in Example 2 (500L pyrrolidone washing kettle), react at 150℃~155℃ for about 5~7 hours, get the crude propiconazole, filter, evaporate pyrrolidone, add 1800L toluene and 500L water to wash, separate layers to remove water, evaporate toluene , Then enter the high vacuum distillation process distillation (see embodiment 4 for details), obtain propiconazole.
[0046] Table five
[0047] The results of five consecutive batches of reaction without micro-negative pressure dehydration
[0048] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com