Novel method for preparing clopidogrel and slat thereof
A clopidogrel and new method technology, applied in the field of preparation of clopidogrel, can solve the problems of complex reaction, low total yield and purity, and achieve easy purification, improved yield and purity, and improved conversion rate and optical purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
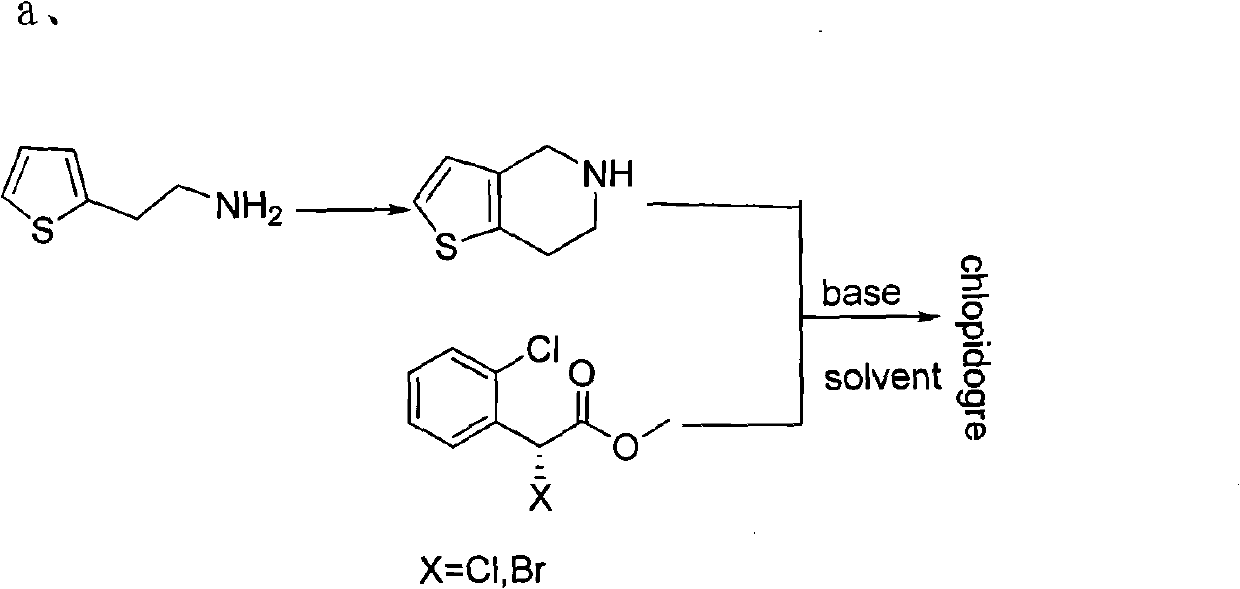
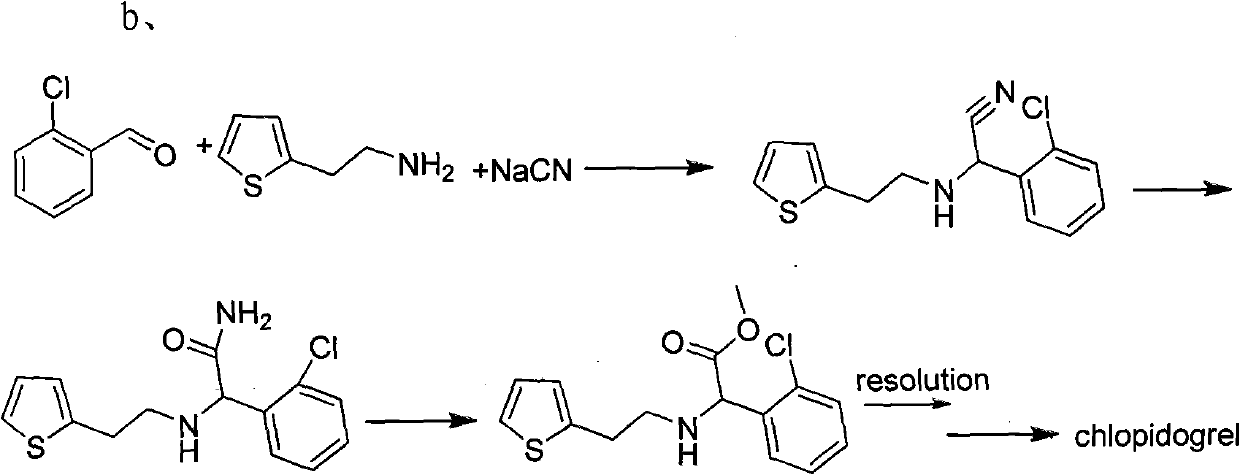
Method used
Image
Examples
Embodiment 1
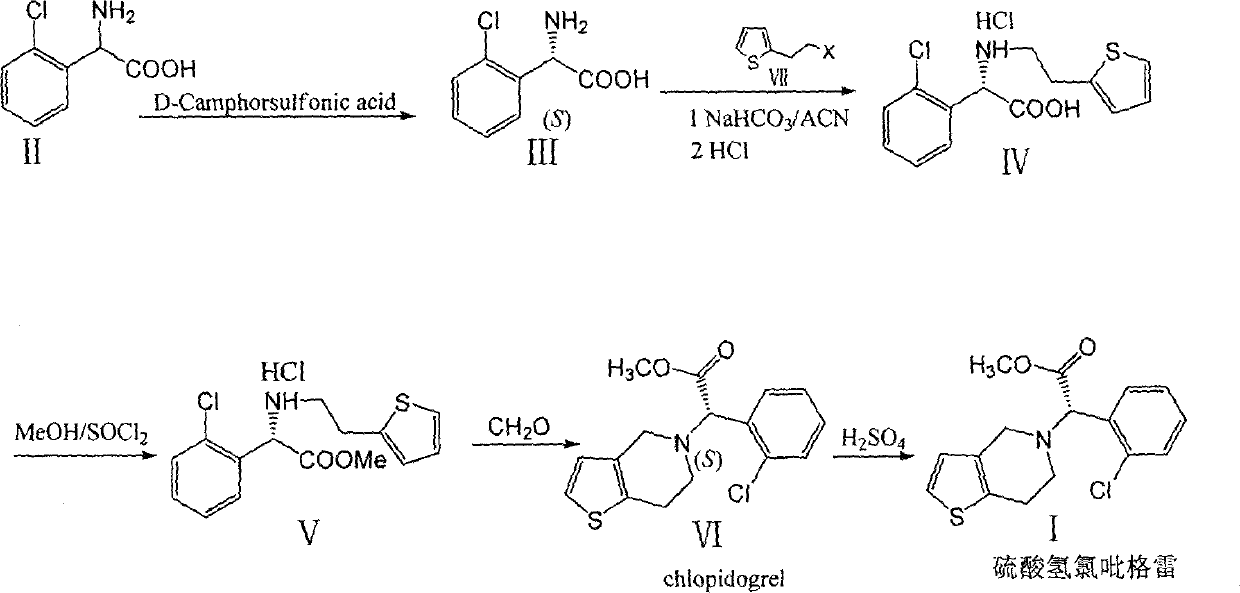
[0030] Embodiment 1: the preparation of (S)-(+)-o-chlorophenylglycine (III)
[0031] In a 250mL single-port reaction equipped with magnetic stirring, add 10.0g (0.0539mol) o-chlorophenylglycine and 13.8g (0.0595mol) D-camphorsulfonic acid, then add 100mL water and heat to 65°C to dissolve , kept stirring for 0.5 hours and then lowered to 45°C for filtration. The filtrate was lowered to -10°C for 6 hours, filtered, and the filter cake was washed with isopropanol (15 mL×2). The mother liquor was concentrated to 50 mL, recrystallized at -10°C, filtered, washed and combined to obtain white solids, and dried to obtain 9.8 g of the product with a purity of 99%, e.e=95%. The solid was added into 30 mL of water, heated to dissolve, frozen to crystallize, filtered, washed with 10 mL of water, and dried to obtain 9.0 g of refined product with a purity of 99.5%, e.e=99%. The two mother liquors were combined and concentrated to dryness to obtain impure (R)-(+)-o-chlorophenylglycine camp...
Embodiment 2
[0037] Example 2: Preparation of (S)-(+)-α-(2-thienylethylamino)-(2-chlorophenyl)acetic acid hydrochloride (IV)
[0038] Take 18.6g (0.1mol) of (S)-(+)-o-chlorophenylglycine and add it to a 250mL three-neck reaction flask with magnetic stirring, then add 100mL of anhydrous acetonitrile to dissolve, and then add 21g (0.25mol) of bicarbonate Sodium and 42g (0.15mol) α-thiophenethanol p-toluenesulfonate, start stirring, and raise the temperature to 80°C for 48 hours. After filtration, the filtrate was concentrated to obtain an oil; the filter cake was washed with ethyl acetate (50 mL×3). Dissolve the previously obtained oil in ethyl acetate as washing liquid, and pass hydrogen chloride gas for 20 minutes under rapid stirring, and a large amount of white solids will precipitate out. After stopping the flow of hydrogen chloride gas, cool to 0°C and keep warm for 2 hours to filter, and filter the cake with a small amount of diethyl ether. washing. The mother liquor was concentrate...
Embodiment 3
[0039] Embodiment 3: Preparation of α-thiophene ethanol p-toluenesulfonate (VII)
[0040] Take 12.7g (0.1mol) of α-thiophene ethanol and put it into a 250mL single-port reaction with magnetic stirring, add 25mL of dichloromethane and 21mL (0.15mol) of triethylamine, and cool to 0°C. Another 21g (0.12mol) of p-toluenesulfonic acid was dissolved in 40mL of dichloromethane, and the solution was slowly dropped into the reaction flask. After dripping, rise to 35°C and react for 5 hours. TLC detects that α-thiophene ethanol disappears, then adds 0.9g sodium bicarbonate and 20mL water, and stirs for another hour. After TLC detects that excess p-toluenesulfonyl chloride is hydrolyzed, cool to room temperature, and then 30 mL of water was added, stirred for 10 minutes, and the organic phase was washed once with saturated brine after liquid separation. The organic phase was dried over anhydrous sodium sulfate, concentrated, and cooled to obtain 26.8 g of a tan solid with a content of 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com