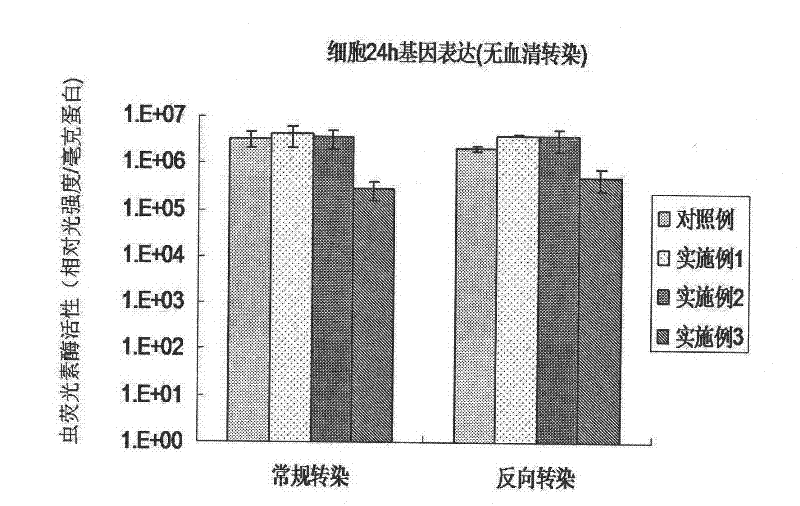
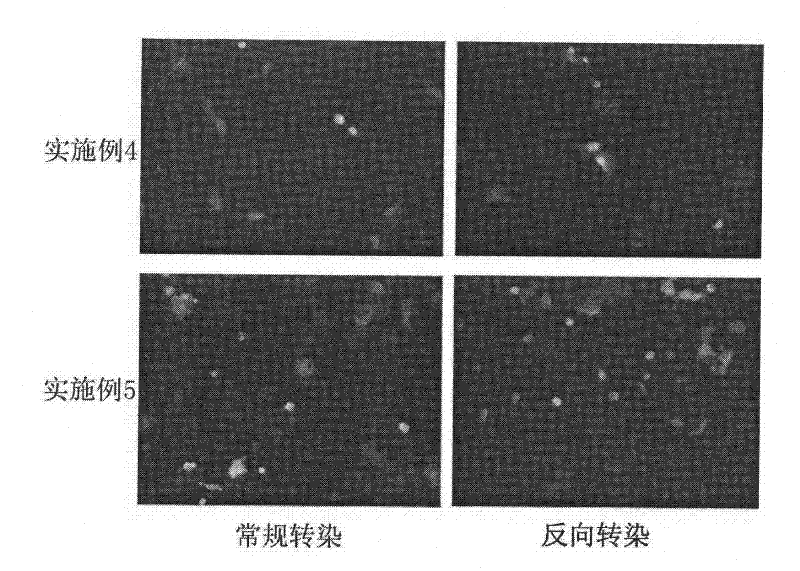
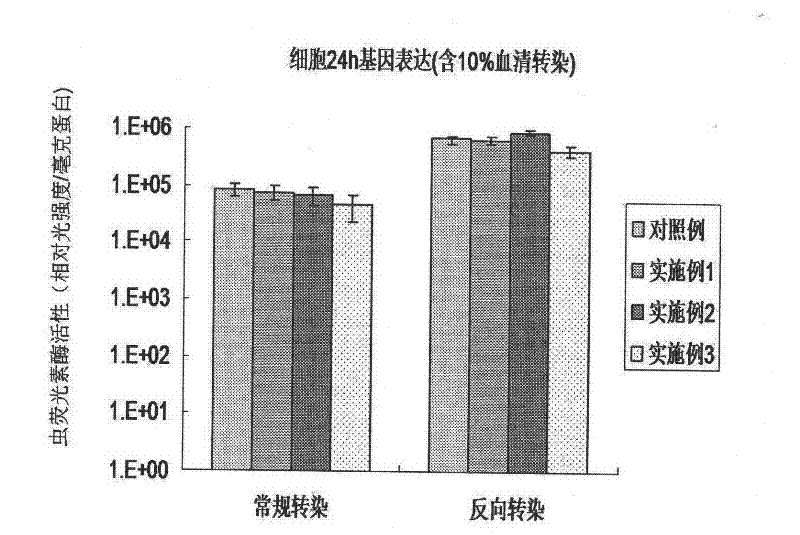
Reverse non-viral vector gene transfection method
A non-viral vector and gene transfection technology, which is applied in the field of reverse non-viral vector gene transfection, can solve the problems of poor controllability and short time, and achieve the effects of high efficiency, simple operation and good transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Apply an aqueous polymer compound solution with a concentration of PEG (Mw=3400) of 2 mg / ml and a concentration of fibronectin of 20 μg / ml to a 24-well cell culture plate (manufactured by Corning, USA), and remove after incubation for 4 hours. The liquid was washed twice with PBS buffer solution (that is, phosphate buffer solution with a Tween-20 concentration of 0.05% by mass and a pH of 7.4) to prepare a surface-modified cell culture plate.
[0036] (2) Using chitosan as a non-viral carrier, mix it with plasmid DNA containing a gene encoding luciferase (ie, pGL3 plasmid DNA, Institute of Pharmacology, Zhejiang University) to prepare a complex of chitosan and DNA.
[0037](3) The complex of chitosan and DNA was added dropwise to the above-mentioned surface-modified cell culture plate, and after incubation for 2 hours, human cervical cancer HeLa cells were added for transfection.
Embodiment 2
[0039] (1) Soak the hyaluronic acid scaffold in a polymer with a concentration of 60 μg / ml of PLL (Mw=100,000) and a polypeptide containing RGD sequence (GRGDSP, synthesized by Shanghai Sangon Bioengineering Co., Ltd.) In the compound aqueous solution, the liquid was removed after incubation for 1 h, and washed twice with PBS buffer solution to prepare the surface-modified hyaluronic acid scaffold.
[0040] (2) Lipofectamine2000 (Invitrogen Company) was used as a non-viral vector and mixed with pGL3 plasmid DNA to prepare a complex of Lipofectamine2000 and DNA.
[0041] (3) The complex of Lipofectamine2000 and DNA was added dropwise onto the surface-modified hyaluronic acid scaffold, incubated for 0.5 h, and then added to human liver cancer HepG2 cells for transfection.
Embodiment 3
[0043] (1) The concentration of anionized gelatin (Mw=50,000) is 500 μg / ml, and the concentration of protamine (Sigma company) is 200 μg / ml of polymer compound aqueous solution coated on the cell culture dish (produced by Hangzhou Shengyou Company) ), after incubation for 12 h, the liquid was removed, washed twice with PBS buffer solution, and the surface-modified cell culture dish was prepared.
[0044] (2) to HD (produced by Roche) as a non-viral vector, prepared by mixing with pGL3 plasmid DNA Complexes of HD and DNA.
[0045] (3) Will The complex of HD and DNA was added dropwise onto the above-mentioned surface-modified cell culture dish, and after incubation for 5 hours, leukemia K562 cells were added for transfection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com