Niobium-doped lithium titanate anode material for lithium ion battery and method for preparing same
A technology for lithium-ion batteries and negative electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve problems that are not conducive to large-scale industrial production, complex and changeable processes, and excessive energy consumption, and achieve considerable reversible capacity, reversible Good controllability and stable cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
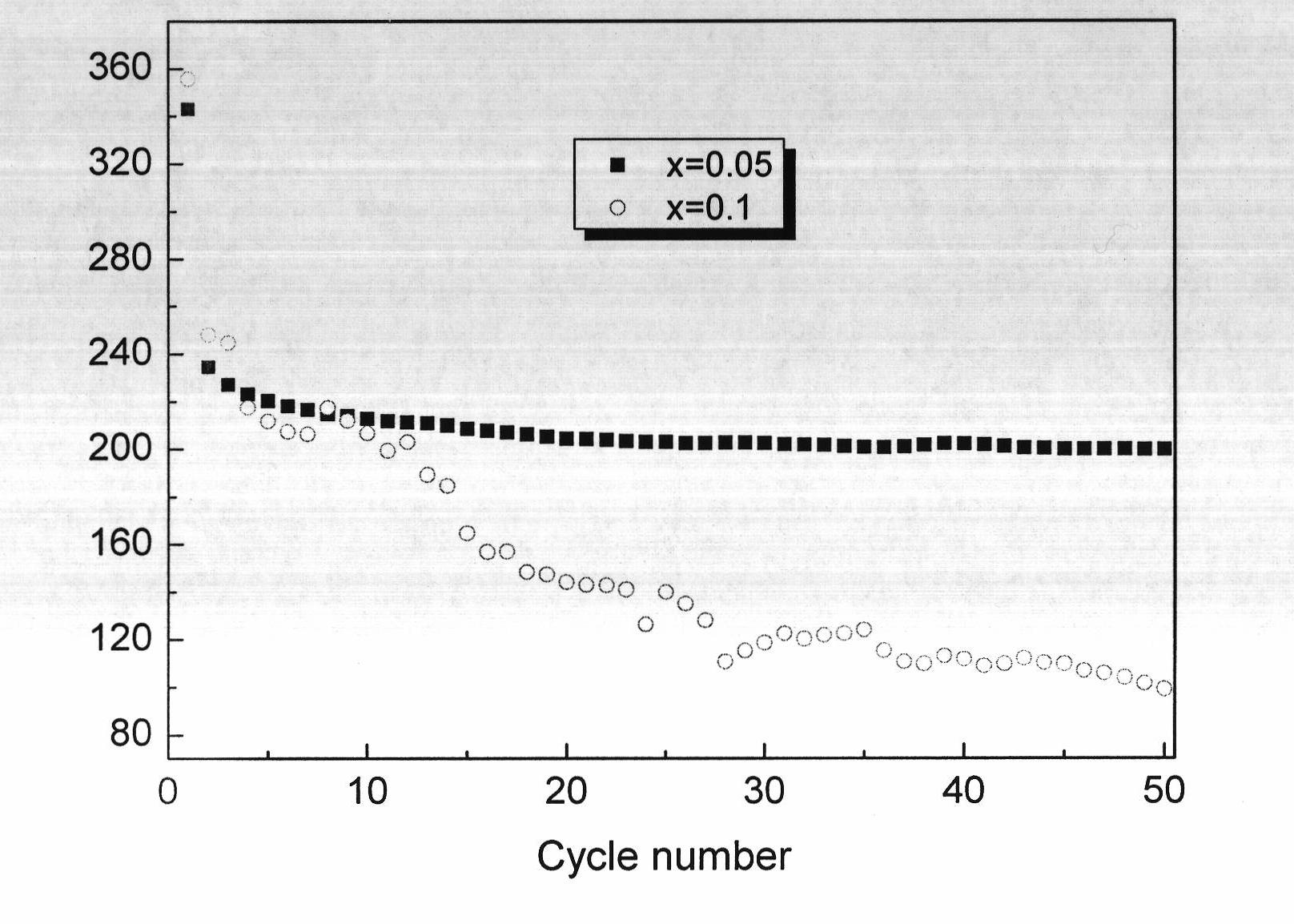
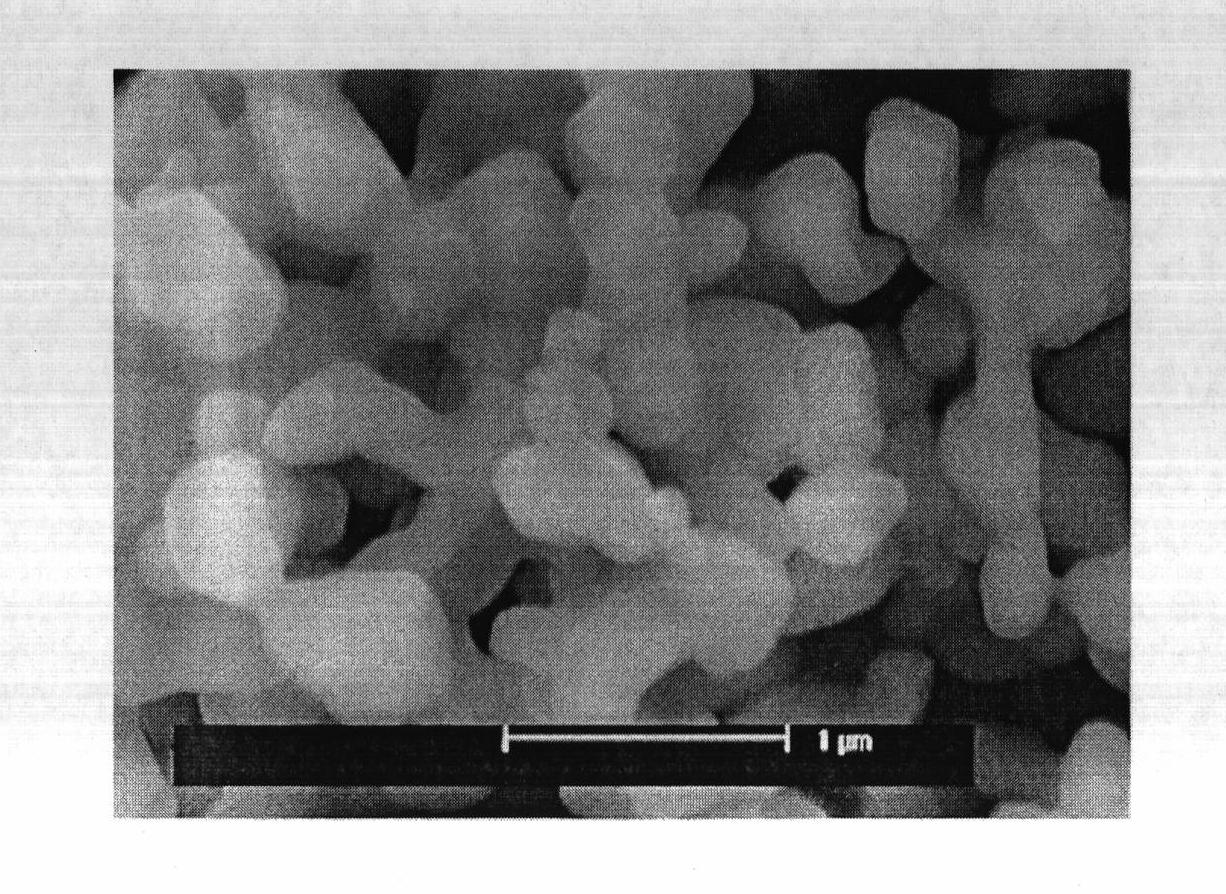
[0020] Embodiment 1: with 0.2mol lithium carbonate, 0.495mol TiO 2 (Anatase type) and 0.0025mol niobium pentoxide are mixed, then put into a ball mill and milled for 8 hours to make it evenly mixed, then put the final mixture into a muffle furnace, react at 850°C for 24 hours, and then cool naturally to room temperature, that is Li 4 Ti 4.95 Nb 0.05 o 12 . X-ray powder diffraction analysis indicated that the resulting Li 4 Ti 4.95 Nb 0.05 o 12 It is a pure phase, without any other impurity phase, and has high crystallinity. According to scanning electron microscope analysis, the particle size of the obtained product is uniform and consistent, and the particle size is 200-300nm. The resulting product was used as an electrode material, and was assembled into an experimental button lithium-ion battery in an argon-filled glove box. The charge-discharge cycle was performed between 0-2V at a rate of 0.1C, and Li 4 Ti 4.95 Nb 0.05 o 12 The first discharge capacity is 343...
Embodiment 2
[0021] Embodiment 2: with 0.2mol lithium carbonate, 0.49mol TiO 2 (Anatase type), 0.005mol niobium pentoxide mixed, and then put into a ball mill for ball milling for 8 hours to make it evenly mixed, then put the final mixture into a muffle furnace, react at 850°C for 24 hours, and then cool naturally to room temperature, that is Li 4 Ti 4.9 Nb 0.1 o 12 . X-ray powder diffraction analysis indicated that the resulting Li 4 Ti 4.9 Nb 0.1 o 12 Contains a small amount of Nb 2 o 5 Impurities. According to scanning electron microscope analysis, the particle size of the obtained product is uniform and consistent, and the particle size is 200-300nm. The resulting product was used as an electrode material and assembled into an experimental button lithium-ion battery in an argon-filled glove box. The charge-discharge cycle was performed between 0-2V at a rate of 0.1C, and Li 4 Ti 4.9 Nb 0.1 o 12 The first discharge capacity is 355mAh·g -1 , the second discharge capacity ...
Embodiment 3
[0022] Embodiment 3: with 0.4mol lithium acetate, 0.495mol TiO 2 (Anatase type), 0.0025mol niobium pentoxide mixed, and then put into a ball mill for 6 hours to make it evenly mixed, then put the final mixture into a muffle furnace, react at 900°C for 22 hours, and then cool naturally to room temperature, that is Li 4 Ti 4.95 Nb 0.05 o 12. The resulting product was used as an electrode material, and was assembled into an experimental button lithium-ion battery in an argon-filled glove box. The charge-discharge cycle was performed between 0-2V at a rate of 0.1C, and Li 4 Ti 4.95 Nb 0.05 o 12 The first discharge capacity is 344mAh·g -1 , the second discharge capacity is 236mAh·g -1 , with a reversible capacity of 199mAh g after 50 cycles -1 , Li 4 Ti 4.95 Nb 0.05 o 12 exhibited excellent electrochemical performance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com