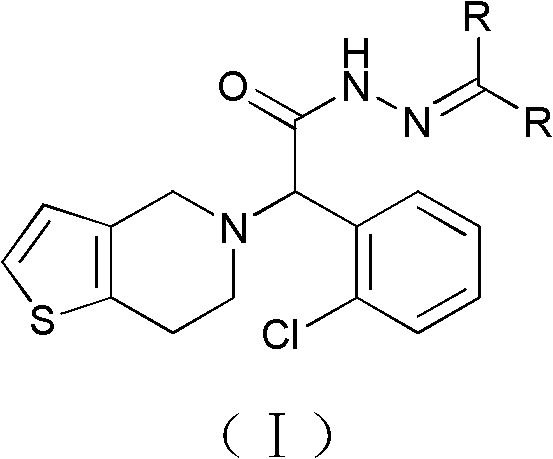
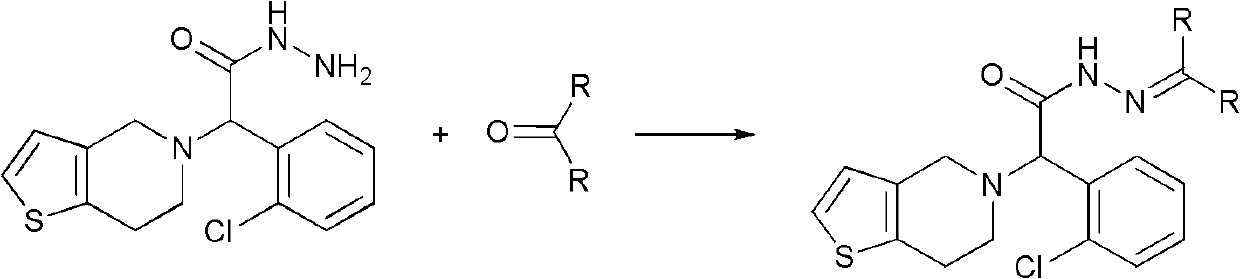
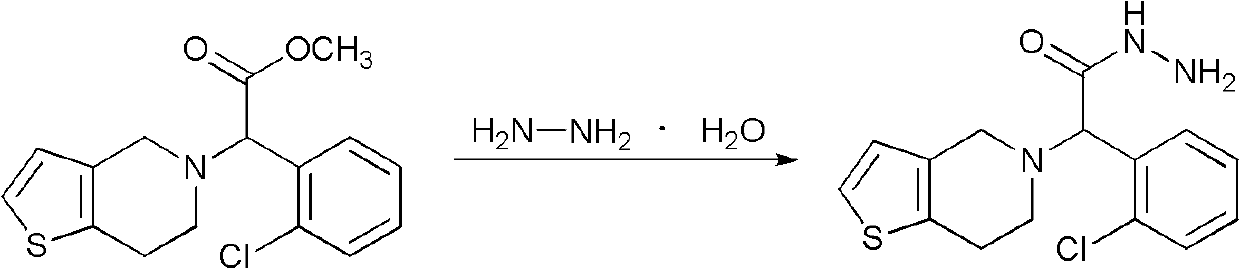Method for preparing anti-platelet aggregation compounds
A compound, hydrazine hydrate technology, applied in the field of chemical pharmacy, can solve the problem of high raw material price, achieve the effect of stable product quality, obvious effect and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 : Synthesis of Intermediate III-1
[0035]
[0036] Heat the solution of intermediate II obtained in the previous step to 50°C, add a mixture of 8.34g (0.1mol) of 60% hydrazine hydrate and 5.81g (0.1mol) of acetone under stirring, and react at 50°C for 1h (the plate layer shows the reaction completely). The reaction was stopped, cooled, and a solid was formed. Filter and dry to obtain 30.3 g of solid product (HPLC: 99.8%), with a yield of 86.7%. Rf=0.41 [single site, developer: ethyl acetate:petroleum ether (60-90°C)=1:1].
Embodiment 2
[0037] Example 2: Synthesis of Intermediate III-2
[0038]
[0039] The solution of intermediate II obtained in the previous step was heated up to 60°C, and a mixture of 6.67g (0.12mol) of 90% hydrazine hydrate and 10.3g (0.12mol) of 3-pentanone was added under stirring, and reacted at 60°C for 0.5h ( Plates show complete reaction). The reaction was stopped, cooled, and a solid was formed. Filter and dry to obtain 33.4 g of solid product (HPLC: 99.0%), yield 87.4%. Rf=0.45 [single site, developing solvent: ethyl acetate:petroleum ether (60-90°C)=1:1].
Embodiment 3
[0040] Example 3: (S)-α,α-[2-Chlorophenyl-2-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)]-N'-[( Dimethyl)methylene]acetylhydrazide
[0041]
[0042]In a 250mL reaction flask equipped with stirring, condenser and thermometer, add 3.5g (0.01mol) of intermediate III-1, add 10mL (0.04mol) of 15% formic acid solution, and add 0.6g (0.02mol) of formaldehyde under stirring . The temperature was raised to 50°C to continue the reaction for 0.5h (the plate showed that the reaction was complete). The reaction was stopped, extracted with 3×10 mL of dichloromethane, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 3.24 g of solid product (HPLC: 99.3%), with a yield of 89.4%. m.p.169.0-170.6°C, Rf=0.37 [single site, developer: ethyl acetate:petroleum ether (60-90°C)=1:1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com