6-methy salicylic acid synthetase transformed by genetic engineering and combinatorial biosynthesis of spirocyclic acetoacetic acid lactone antiboitic
A technology of spirocyclic acetoacetic acid and antibiotics, applied in the field of biotechnology engineering, can solve problems such as limited production prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
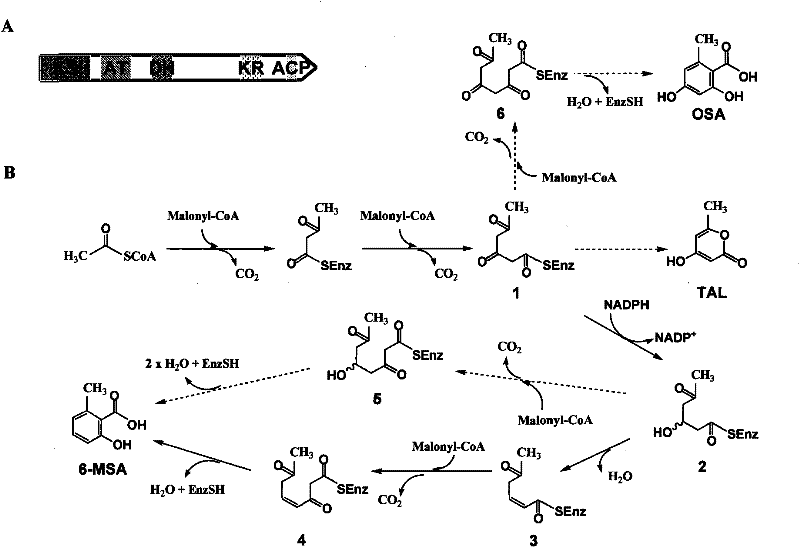
[0043] Example 1 Point mutation and heterologous expression of DH and KR domains of ChlB1
[0044] 1. Acquisition of ChlB1 with point mutations in DH and KR domains
[0045] 1) Mutation of the DH domain
[0046] Inactivate the DH domain of ChlB1, choose to mutate its 947 histidine residue, and mutate it into glycine or phenylalanine. First, take two pairs of designed primers, and the primer sequences are as follows:
[0047] 947 position H is mutated to A
[0048] Upstream: 5’-C TAC CCC GGC AGC GCC ACC ATC AAC GGC ACG-3’
[0049] Downstream: 5’-CGT GCC GTT GAT GGT GGC GCT GCC GGG GTA G-3’
[0050] 947 position H is mutated to F
[0051] Upstream: 5’-C TAC CCC GGC AGC TTC ACC ATC AAC GGC ACG-3’
[0052] Downstream: 5’-CGT GCC GTT GAT GGT GAA GCT GCC GGG GTA G-3’
[0053] Using pAL1084 (a fragment of the wild-type DH domain cloned into pSP72) as a template template, dNTP, DMSO, enzyme-free water, high-fidelity primestar enzyme and its buffer constitute a PCR reaction syst...
Embodiment 2
[0087] Example 2, KR mutated ChlB1 complementary ChlB1 mutants produce new analogs of CHL
[0088] 1. Intergeneric conjunctive transfer between E.coli ET12567 and Streptomyces antibioticus DSM40725(ΔchlB1)
[0089] Pick a single colony from the transformed E.coli ET12567 culture plate and transfer it to a test tube for overnight culture, pipette 0.5ml of the bacterial solution into 25ml LB, and culture it in a shaker at 37°C until OD 600 It is 0.3-0.4, or 0.4-0.6. The bacteria were collected by centrifugation, washed twice with an equal volume of LB medium, and the bacteria were collected by centrifugation and suspended in 1ml of LB medium. as a DNA donor.
[0090] Take out the spore suspension (3 × 10 9 cells / mL), centrifuge at 8,000rpm for 3 minutes to remove the supernatant, then wash twice with 0.5ml TES buffer (0.05M, pH 8.0), resuspend in 500μl TES buffer (0.05M, pH 8.0), heat shock at 50°C for 10 minutes To stimulate spore germination, then add 500μl TSB, mix well, ...
Embodiment 3
[0096] Example 3, fermentation isolation and purification of new compounds of mutant strains, structural identification and determination of biological activity
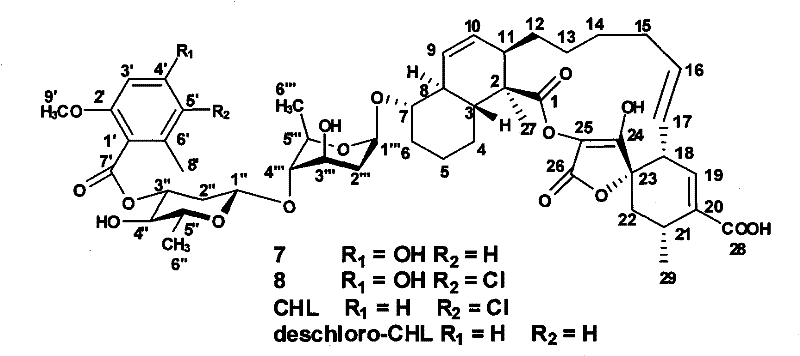
[0097] When the mutant strain was fermented in a small amount, two possible new compounds 7 and 8 were detected by HPLC and LC-MS. In order to identify the structures of these two compounds, we carried out a large amount of fermentation on the mutant strain. See Example 2 for the fermentation method. After the culture medium is freeze-dried, mash it and add an equal volume of anhydrous methanol, sonicate for 10 minutes (ultrasound for 10 seconds, interval of 50 seconds), stir with a stirrer for 1 hour, filter (or centrifuge) to collect methanol, and rotate to remove methanol , dispersed with 1 / 5 volume of water, adjusted to pH 2.0-3.0, extracted three times with an equal volume of ethyl acetate, and dried under reduced pressure to obtain a dark brown paste. The first step of the paste is coarse separation, mix the pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com