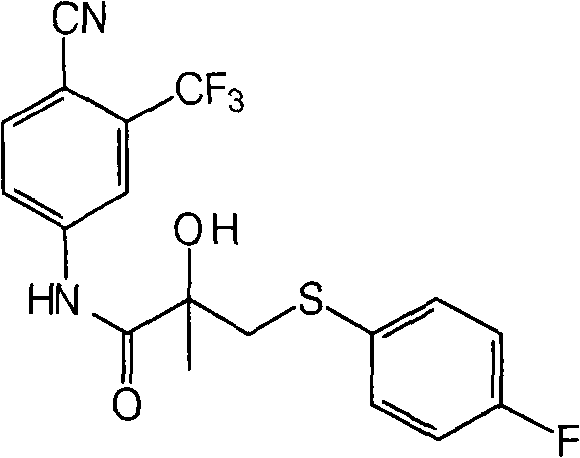
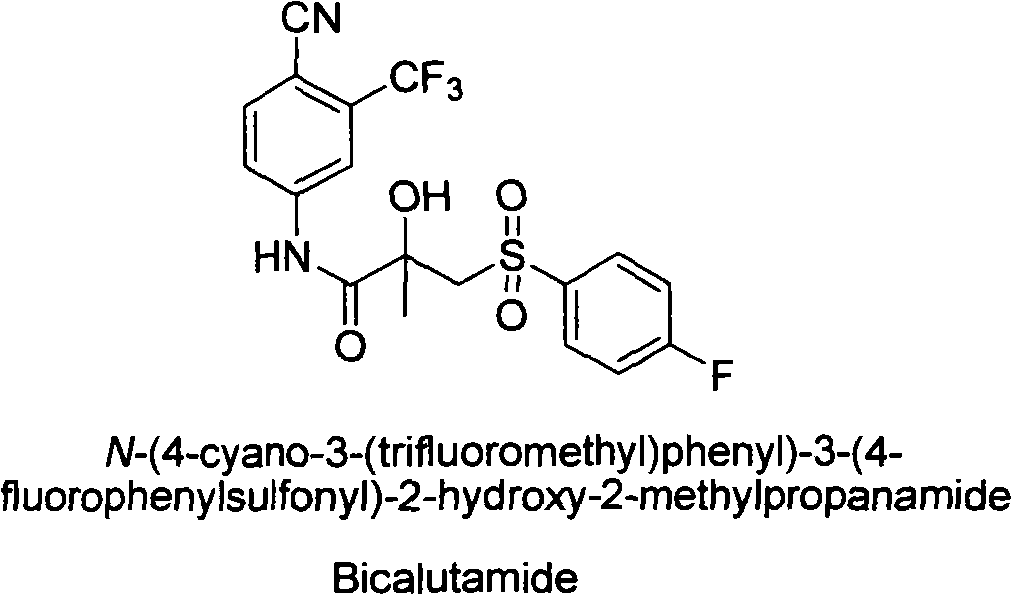
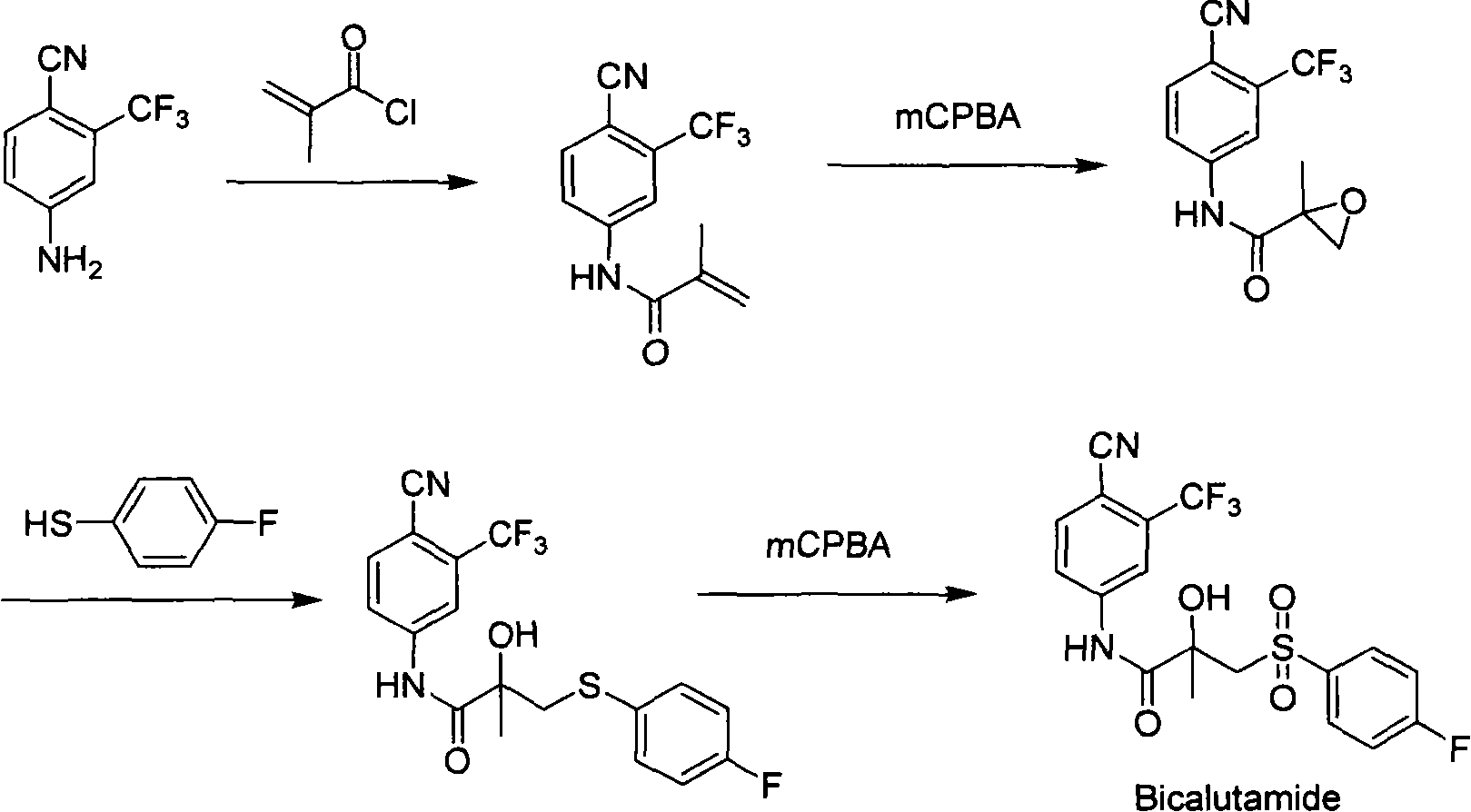
New process of preparing bicalutamide by utilizing air for oxidizing bicalutamide thioether intermediate
A technology of carbalutamide sulfide and bicalutamide, which is applied to the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., can solve the problems of complex processing and high production costs, and achieve easy reaction, cost reduction, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The bicalutamide sulfide intermediate (10.0g, 0.025mol) was dissolved in ethyl acetate (80ml), and isobutyraldehyde (7.2g, 9.1ml, 0.1mol) was added under stirring at room temperature, and the catalyst Co(acac) 3 (0.05g), free radical initiator 30%, aqueous hydrogen peroxide (0.1ml). Keep the temperature at 25-30° C., react overnight, and TLC detects that the starting material disappears. In order to separate the gained product from the reaction system, add water (80ml), and the saturated sodium sulfite solution is slowly dripped in until the reaction solution makes the starch potassium iodide test paper colorless. The layers were allowed to stand, and the layers were separated, and the aqueous layer was extracted with ethyl acetate (3×50ml). The organic layers were combined, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain a white solid. Recrystallization in acetone / water gave 10.5 g of white bicalutamide crystals. The yie...
Embodiment 2
[0042] Bicalutamide sulfide intermediate (10.0g, 0.025mol) was dissolved in dichloromethane (100ml), pivalaldehyde (8.6g, 10.8ml, 0.1mol) was added under stirring at room temperature, catalyst Co(acac) 3 (0.05g), free radical initiator 30%, aqueous hydrogen peroxide (0.1ml). Keep the temperature at 25-30° C., react overnight, and TLC detects that the starting material disappears. In order to separate the gained product from the reaction system, add water (80ml), and the saturated sodium sulfite solution is slowly dripped in until the reaction solution makes the starch potassium iodide test paper colorless. Cool to 10°C, filter, and wash the filter cake with water and a little dichloromethane to obtain 10.7 g of white bicalutamide solid. The yield is 99.1%, the melting point is 191-193°C, and the HPLC content is 99%.
Embodiment 3
[0044] The bicalutamide thioether intermediate is oxidized to synthesize bicalutamide, and isobutyraldehyde (7.2g, 9.1ml, 0.1mol) is replaced with pivalaldehyde (8.6g, 10.8ml, 0.1mol), and other operations are the same as in the examples 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com