Fluorescent material and preparation method thereof
A technology of fluorescent materials and compounds, applied in the field of luminescent materials, can solve problems such as energy loss, color reabsorption, and affecting the service life of white light LEDs, and achieve the effect of reducing difficulty and increasing service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The present invention also provides a method for preparing a fluorescent material, the fluorescent material has an atomic ratio composition represented by the general formula (I):
[0055] m 9(1-x-y) Ln(PO 4 )7 :Eu x , Mn y
[0056] (I)
[0057] Its preparation method includes:
[0058] Grinding and mixing the M-containing compound, the Ln-containing compound, the Eu-containing compound, the Mn-containing compound and ammonium dihydrogen phosphate and / or diammonium hydrogen phosphate to obtain a mixture;
[0059] Calcining the mixture under a reducing atmosphere to obtain a fluorescent material;
[0060] The M is one or more of Mg, Ca, Sr or Ba, and the M-containing compound is one or more of the oxide, hydroxide or carbonate of M;
[0061] Ln is one or more of La, Lu, Gd, Dy, Tb, Y, Ce, Pr, Nd, Sm, Ho, Er, Tm or Yb, and the compound containing Ln is an oxide of Ln, containing One or more of hydroxides of Ln or carbonates containing Ln;
[0062] The Eu-containi...
Embodiment 1
[0071] Weigh 0.4279gCaCO 3 , 0.0906gGd 2 o 3 , 0.4026gNH 4 h 2 PO 4 , 0.0079gEu 2 o 3 and 0.0207gMnCO 3 , thoroughly grind and mix the above-mentioned substances, place them in a corundum crucible, bake them in a high-temperature furnace at 1200°C in a CO atmosphere for 4 hours, take them out when cooled to 1000°C, and after grinding and dispersing, the composition of Ca is obtained. 8.55 Gd(PO 4 ) 7 :Eu 0.01 , Mn 0.04 fluorescent material.
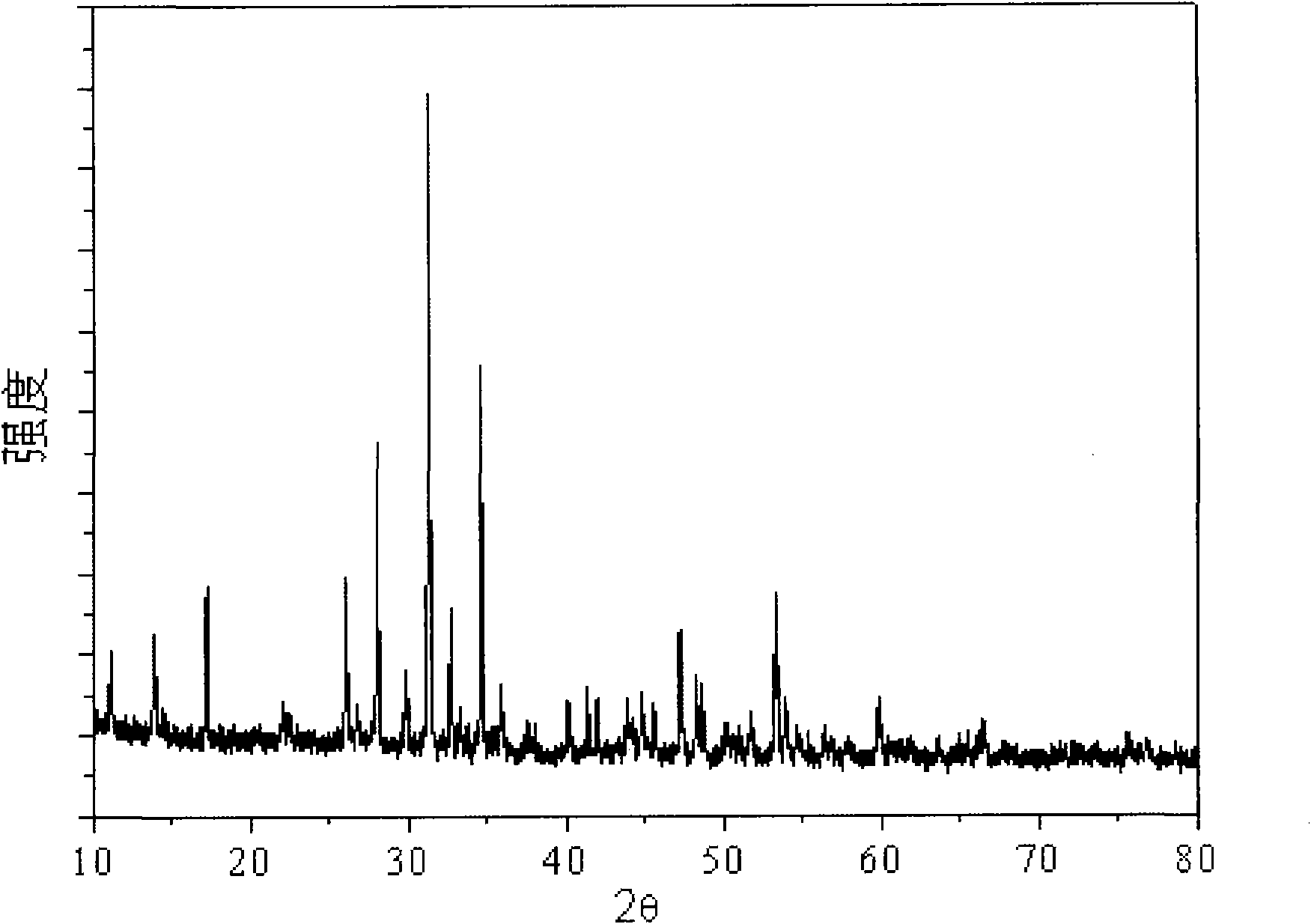
[0072] Carry out X-ray powder diffraction (XRD) to the fluorescent material prepared in this embodiment with Bruker D8Focus type diffractometer, test condition is: CuKα radiation, λ=0.15405nm, acceleration voltage and emission current are respectively 40kV and 40mA, scanning range: 2θ =10-80°; test results see figure 1 , figure 1 For the XRD spectrogram of the fluorescent material prepared in Example 1 of the present invention, by figure 1 It can be seen that the fluorescent material obtained in the embodiment of the presen...
Embodiment 2
[0075] Weigh 0.4279gCaCO 3 , 0.0565gY 2 o 3 , 0.4026gNH 4 h 2 PO 4 , 0.0109gEu 2 (CO 3 ) 3 and 0.0207gMnCO 3 , carry out thorough grinding and mixing, put it into a corundum crucible, put it into a high-temperature furnace and bake it in a CO atmosphere at 1200 ° C for 4 hours, take it out when it is cooled to 1000 ° C, and after grinding and dispersing, the composition of Ca is obtained. 8.55 Y(PO 4 ) 7 :Eu 0.01 , Mn 0.04 fluorescent material.
[0076] Carry out X-ray powder diffraction (XRD) to described fluorescent material with Bruker D8Focus type diffractometer, the result shows that the fluorescent material that the embodiment of the present invention obtains is a single substance, has realized Eu and Mn in Ca 8.55 Y(PO 4 ) 7 Doping in.
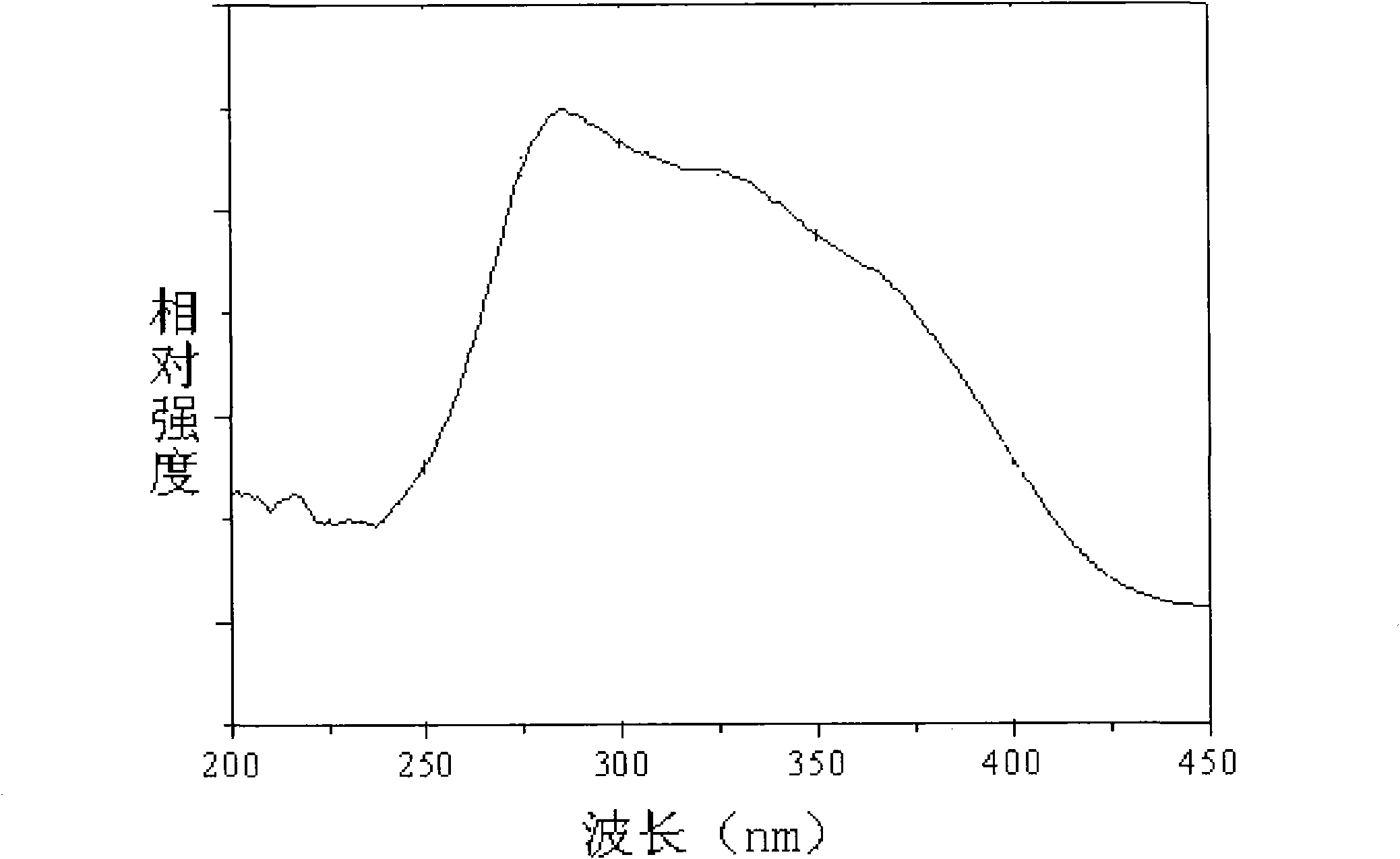
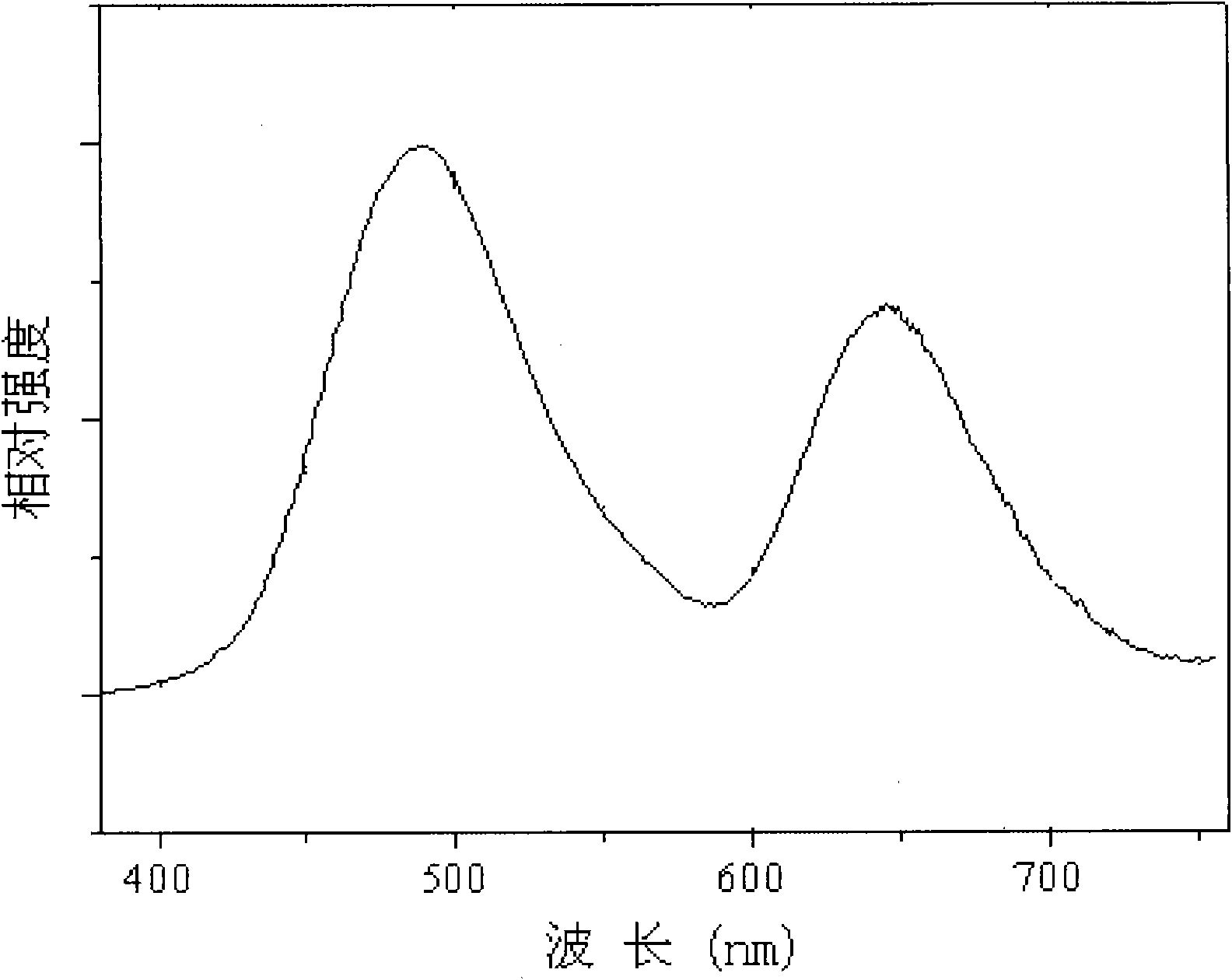
[0077] Excitation and emission tests were carried out on the fluorescent material with a Hitachi F-7000 fluorescence spectrometer, and a 150W xenon lamp was used as the excitation light source. Excited by ultraviolet li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com