Preparation method and application of cerium-zirconium composite oxide catalyst loaded with copper oxide
A composite oxide and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of long consumption time, low low temperature oxidation activity of CO, The catalyst process is cumbersome and other problems, to achieve the effect of simple preparation process, high anti-sintering ability and thermal stability, and short working hours
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
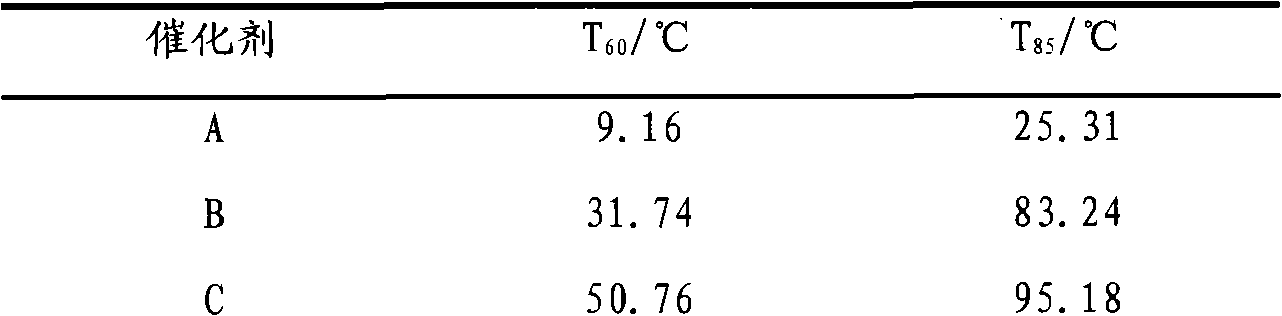
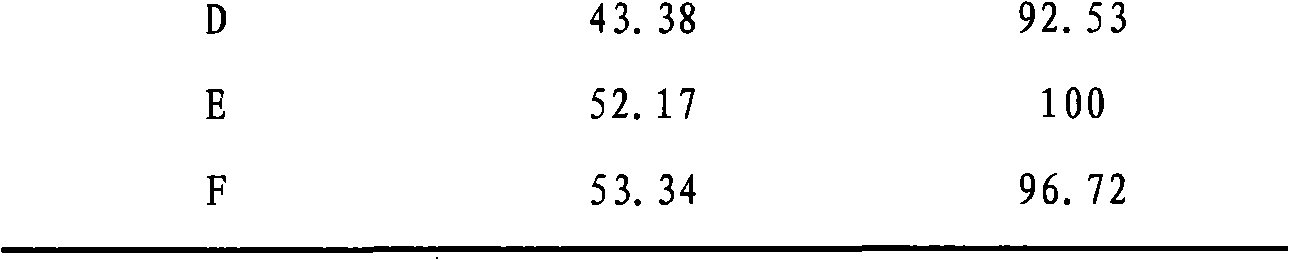
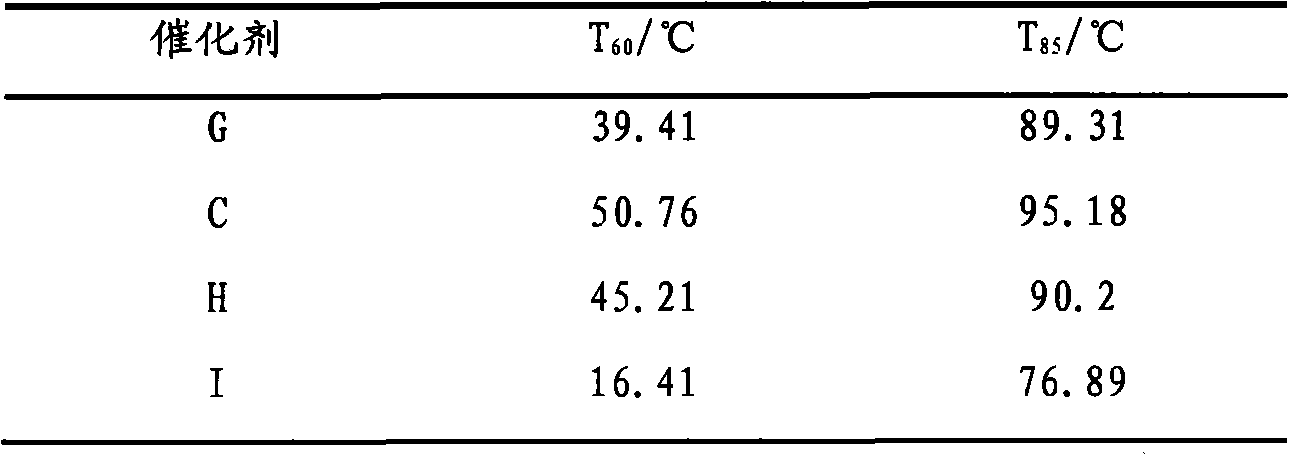
[0026] 5.211g Ce(NO 3 ) 3 ·6H 2 O, 5.1506g Zr(NO 3 ) 4 ·5H 2 O and 0.1183g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 200mL deionized water, and added dropwise to 100mL ammonia solution with a mass fraction of 5%-8% at a rate of 0.8-1.2mL / min under stirring at 600-900r / min. After the dropwise addition was completed, stirring was continued for 1 h. Carry out microwave heating and reflux at a stirring speed of 700-900 r / min, and stop heating until pH=6-7. Filter the mixture when it is cooled to 80-90°C, and wash the filter cake twice with absolute ethanol that is 2 to 3 times the mass of the filter cake. The filter cake was heated and dried in a microwave oven, and baked in a muffle furnace at 600° C. for 2 hours to prepare catalyst A, in which the content of CuO in the catalyst was 2%.
Embodiment 2
[0028] 3.9083g Ce(NO 3 ) 3 ·6H 2 O, 2.5753g Zr(NO 3 ) 4 ·5H 2 O and 0.4027g Cu(NO 3 ) 2 ·3H 2O was dissolved in 150 mL of deionized water, and added dropwise to 70 mL of ammonia solution with a mass fraction of 5% to 8% at a rate of 0.8 to 1.2 mL / min under stirring at 600 to 900 r / min. After the dropwise addition was completed, stirring was continued for 1 h. Carry out microwave heating and reflux at a stirring speed of 700-900 r / min, stop heating until pH=6-7. Filter the mixture when it is cooled to 80-90°C, and wash the filter cake twice with absolute ethanol that is 2 to 3 times the mass of the filter cake. The filter cake was heated and dried in a microwave oven, and baked in a muffle furnace at 600° C. for 2 hours to prepare catalyst B, in which the content of CuO in the catalyst was 10%.
Embodiment 3
[0030] 3.9083g Ce(NO 3 ) 3 ·6H 2 O, 2.5753g Zr(NO 3 ) 4 ·5H 2 O and 0.906g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 160 mL of deionized water, and added dropwise to 100 mL of ammonia solution with a mass fraction of 5% to 8% at a rate of 0.8 to 1.2 mL / min under stirring at 600 to 900 r / min. After the dropwise addition was completed, stirring was continued for 1 h. Carry out microwave heating and reflux at a stirring speed of 700-900 r / min, and stop heating until pH=6-7. Filter the mixture when it is cooled to 80-90°C, and wash the filter cake twice with absolute ethanol that is 2 to 3 times the mass of the filter cake. The filter cake was heated and dried in a microwave oven, and then calcined in a muffle furnace at 600° C. for 2 hours to prepare catalyst C. The content of CuO in the catalyst was 20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com