Method for treating waste acid by gypsum sedimentation, arsenic oxidizing sedimentation and iron salt neutralization and co-precipitation
A gypsum and polluted acid technology, applied in chemical instruments and methods, neutralized water/sewage treatment, oxidized water/sewage treatment, etc. The effect of reducing the amount of slag and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
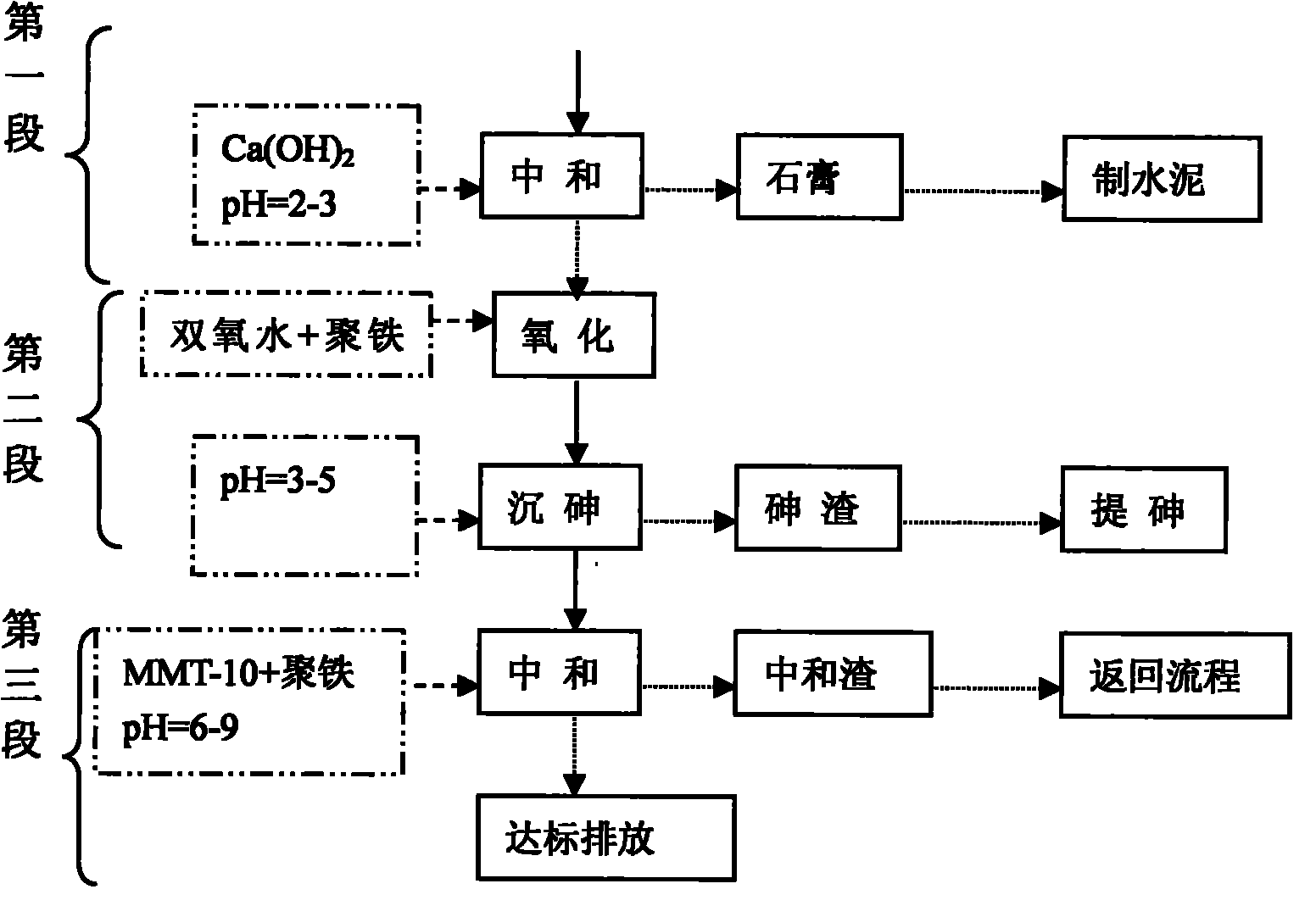
[0042] As shown in Figure 1, the first stage (gypsum process): high arsenic and high acid dirty acid enters the waste acid tank through the waste acid pump into the gypsum reaction tank to react with slaked lime milk for gypsum reaction, and the gypsum reaction liquid enters the concentration tank for precipitation; The clear water enters the regulating tank; the underflow (ie gypsum) enters the centrifugal separator for dehydration to produce gypsum for transportation.
[0043] The second stage (arsenic precipitation process): the gypsum supernatant enters the adjustment tank together with other low-arsenic and low-acid wastewater; the adjustment pump is pumped into the arsenic oxide precipitation reaction tank, polymer iron is added, hydrogen peroxide is added, and the reaction enters the precipitation tank , The supernatant enters the third stage of the process. After the underflow enters the concentration tank and is concentrated, it is pumped into the arsenic slag dehydratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com