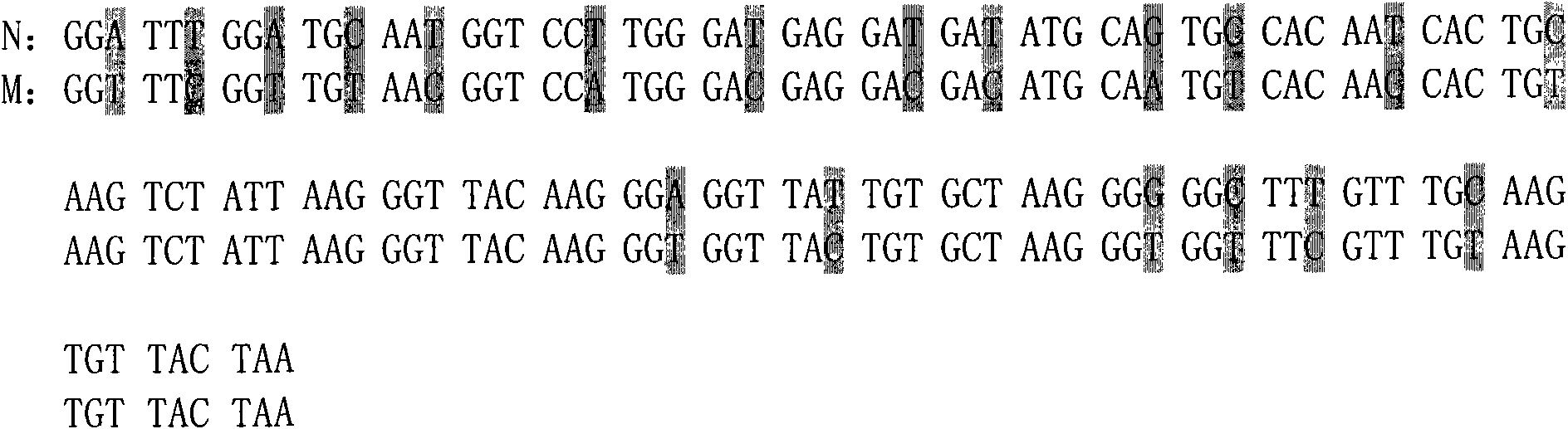Plectasin mature polypeptide dimer fusion protein and preparation method thereof
A peptide dimer, fusion protein technology, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Artificial synthesis of pseudo-sigoldin mature polypeptide dimer fusion protein gene
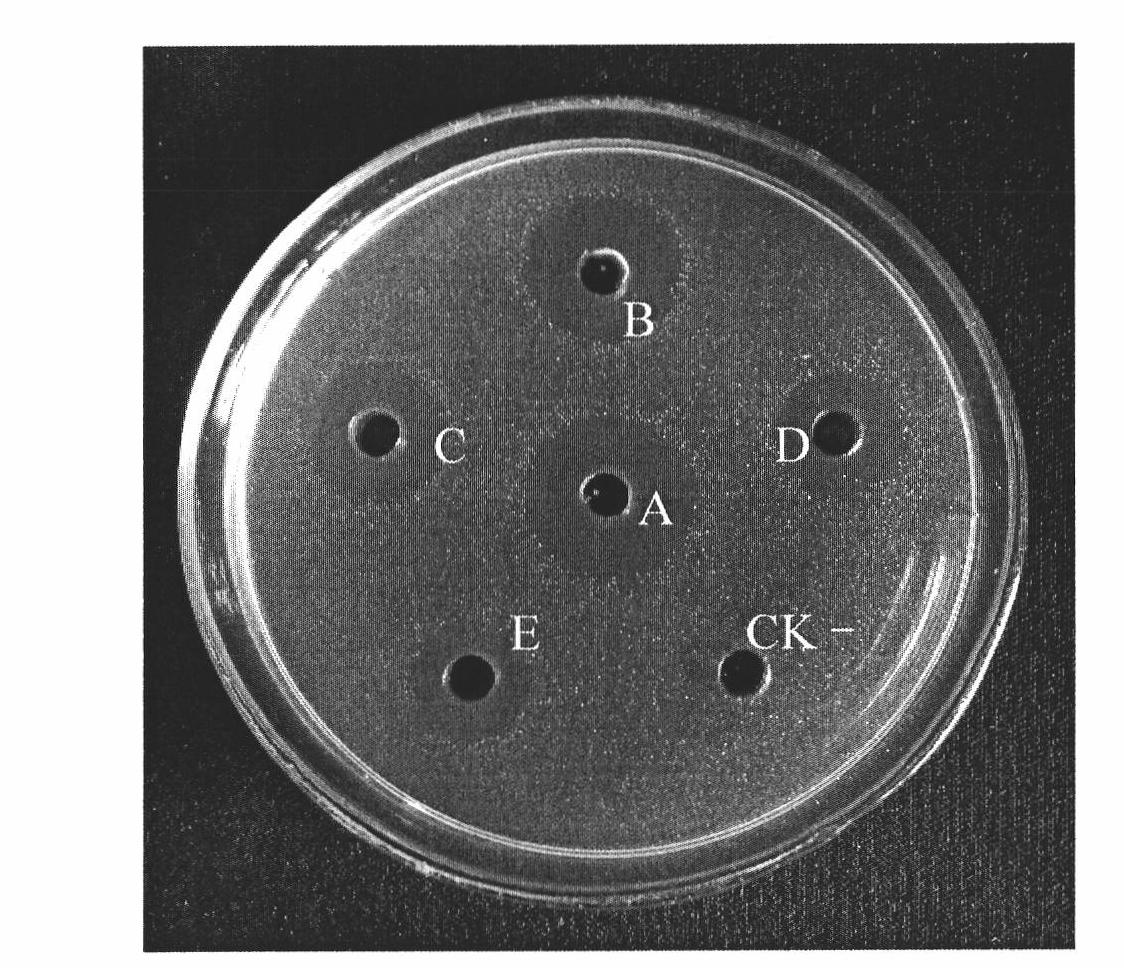
[0060] According to the known full gene sequence (GenBank: AJ964941) of Pseudomycin, according to the codons preferred by Pichia pastoris, without changing its amino acid sequence, the gene encoding the mature polypeptide of Pseudosiglobin was analyzed. Codon optimization. Compared with the unoptimized mature polypeptide gene of the optimized pseudosigladin, 19 nucleotide bases have been changed, involving a total of 19 codons, and the G+C content has changed from the original 45% to 46%. , see the comparison before and after codon optimization of the mature polypeptide gene of pseudosigladin figure 1 , the optimized mature pseudosigantillin gene still encodes the same 40 amino acid residues (see SEQ ID NO: 1), and the molecular weight is about 4.4KDa.
[0061] When artificially synthesizing the fusion protein gene of the mature pseudo-sigantagactin polypeptide dimer, the...
Embodiment 2
[0062] Embodiment 2: Cloning of the fusion protein gene of the mature polypeptide dimer of pseudosigaldin
[0063] The artificially synthesized plpDNA fragment of the mature polypeptide dimer fusion protein gene plpDNA of the above artificially synthesized pEASY-T1 (purchased from Beijing TransGenic Company) was directly inserted into the T site of the plasmid, and according to the method provided by the company, the obtained Bacterial clones containing the plasmid vector plp-T. Through DNA sequencing analysis, it was determined that the pseudosigalcin mature polypeptide dimer fusion protein gene plp contained in it was correct and complete (DNA sequencing was completed by Beijing Biaokai Technology Co., Ltd.).
Embodiment 3
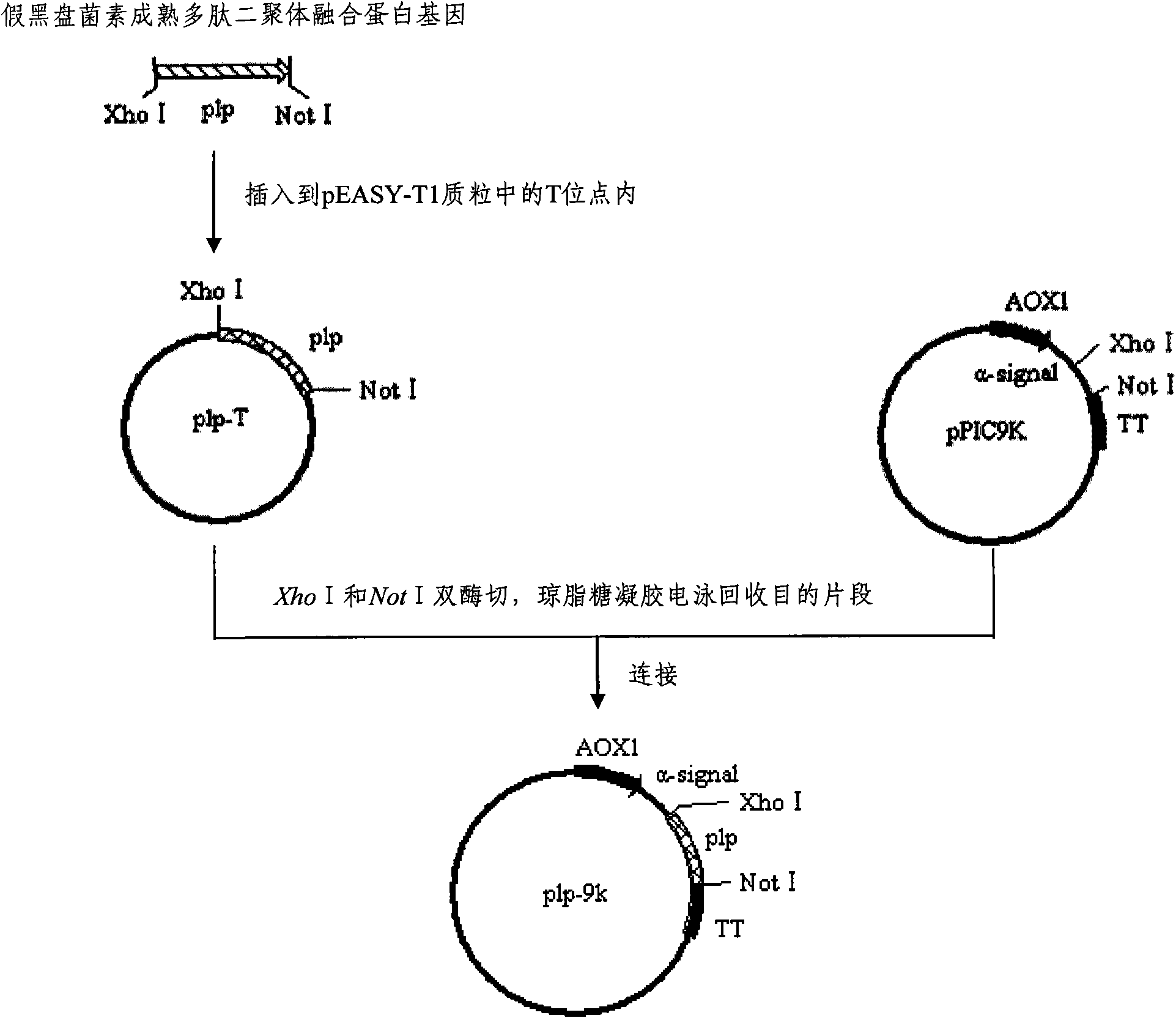
[0064] Embodiment 3: Construction of yeast expression vector plp-9k
[0065] Perform double digestion with restriction endonucleases Xho I and Not I, cut out the pseudo-alphacin mature polypeptide dimer fusion protein gene plp connected to the intermediate plasmid vector plp-T, and run through agarose gel electrophoresis The dimeric fusion protein gene fragment is isolated and recovered. The plasmid pPIC9K was treated with the same restriction endonuclease, and the linearized pPIC9K plasmid DNA was separated and recovered by agarose gel electrophoresis. After the above two DNA fragments are mixed, they are linked together with ligase to obtain the high-efficiency expression vector plp-9k of Pichia pastoris (see figure 2 ). Then, the plasmid expression vector was used to transform Escherichia coli cells DH5α (purchased from GIBCO, USA) so as to replicate and preserve the plasmid.
[0066] Plasmid vector pPIC9K was purchased from Invitrogen, USA. It is a yeast-inducible expr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com