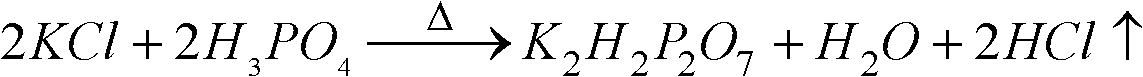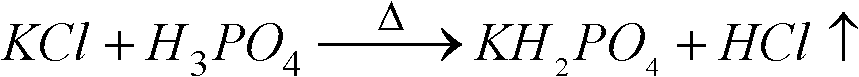Method for producing potassium dihydrogen phosphate with double decomposition method
A technology of potassium dihydrogen phosphate and metathesis, which is applied in the field of production of potassium dihydrogen phosphate by metathesis, can solve the problems of low concentration of by-product hydrochloric acid, low recovery value, prolonging reaction time, etc., so as to increase the concentration of by-product hydrochloric acid, improve The effect of recycling value and reducing production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
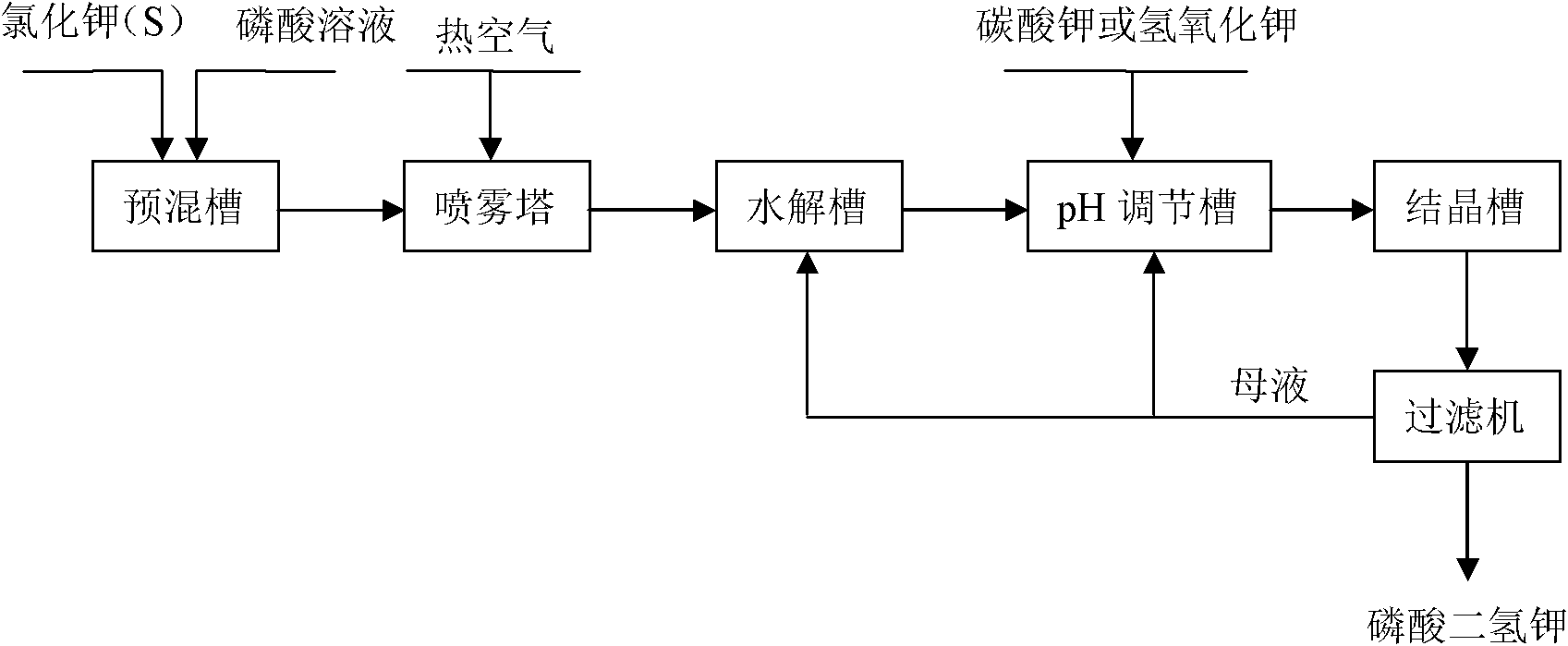
[0034] with P 2 O 5 %=30 phosphoric acid and potassium chloride powder with a particle size of 180 μm to 250 μm are reacted at a molar ratio of P / Cl=1.8 / 1, first make potassium chloride and phosphoric acid fully mixed, and the mixture is injected into the spray tower for reaction, and then The temperature of the tower is 400 °C, the temperature of the outlet is 180 °C, and the temperature of the metathesis reaction is controlled at about 200 °C. 2 O 5 The concentration of %=25 is hydrolyzed at a temperature of 110 ° C, then potassium hydroxide is added to adjust the pH value of the hydrolysis solution to 4.3~4.5, cooling and crystallization to obtain potassium dihydrogen phosphate product, and the crystallization mother liquor is returned to the hydrolysis tank for recycling. The hydrogen chloride gas generated in the spraying process is washed and absorbed with a 25% w / w potassium hydroxide solution to obtain potassium chloride.
[0035] Reaction under this condition, the ...
Embodiment 2
[0039] with P 2 O 5 %=40 phosphoric acid and potassium chloride powder with a particle size of 75 μm to 96 μm are reacted at a molar ratio of P / Cl=1.6 / 1, first make potassium chloride and phosphoric acid fully mixed, and the mixture is injected into the spray tower for reaction, and then The temperature of the tower is 500 °C, the temperature of the outlet is 200 °C, and the temperature of the metathesis reaction is controlled at about 260 °C. 2 O 5 After the concentration of %=35 is hydrolyzed at a temperature of 90 DEG C, potassium hydroxide is added to adjust the pH value of the hydrolysis solution to 4.3~4.7, cooling and crystallization to obtain potassium dihydrogen phosphate product, and the crystallization mother liquor is returned to the pH adjustment tank for recycling. Hydrochloric acid is obtained by washing and absorbing the hydrogen chloride gas generated during the spraying process with water.
[0040] Reaction under this condition, the material conversion rat...
Embodiment 3
[0044] with P 2 O 5 %=40 phosphoric acid and potassium chloride powder with a particle size of 150 μm to 180 μm are reacted with the molar ratio of P / Cl=1.4 / 1. First, the potassium chloride and phosphoric acid are fully mixed, and the mixture is injected into the spray tower for reaction. The temperature of the tower is 500 °C, the temperature of the outlet is 200 °C, and the temperature of the metathesis reaction is controlled at about 260 °C. 2 O5 After the concentration of %=35 is hydrolyzed at a temperature of 90 DEG C, potassium hydroxide is added to adjust the pH value of the hydrolysis solution to 4.3~4.7, cooling and crystallization to obtain potassium dihydrogen phosphate product, and the crystallization mother liquor is returned to the pH adjustment tank for recycling. Hydrochloric acid is obtained by washing and absorbing the hydrogen chloride gas generated during the spraying process with water.
[0045] Reaction under this condition, the material conversion rate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com