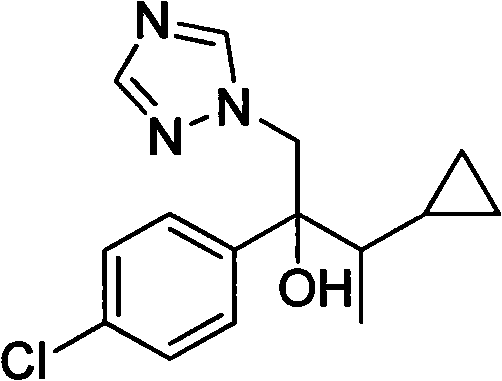
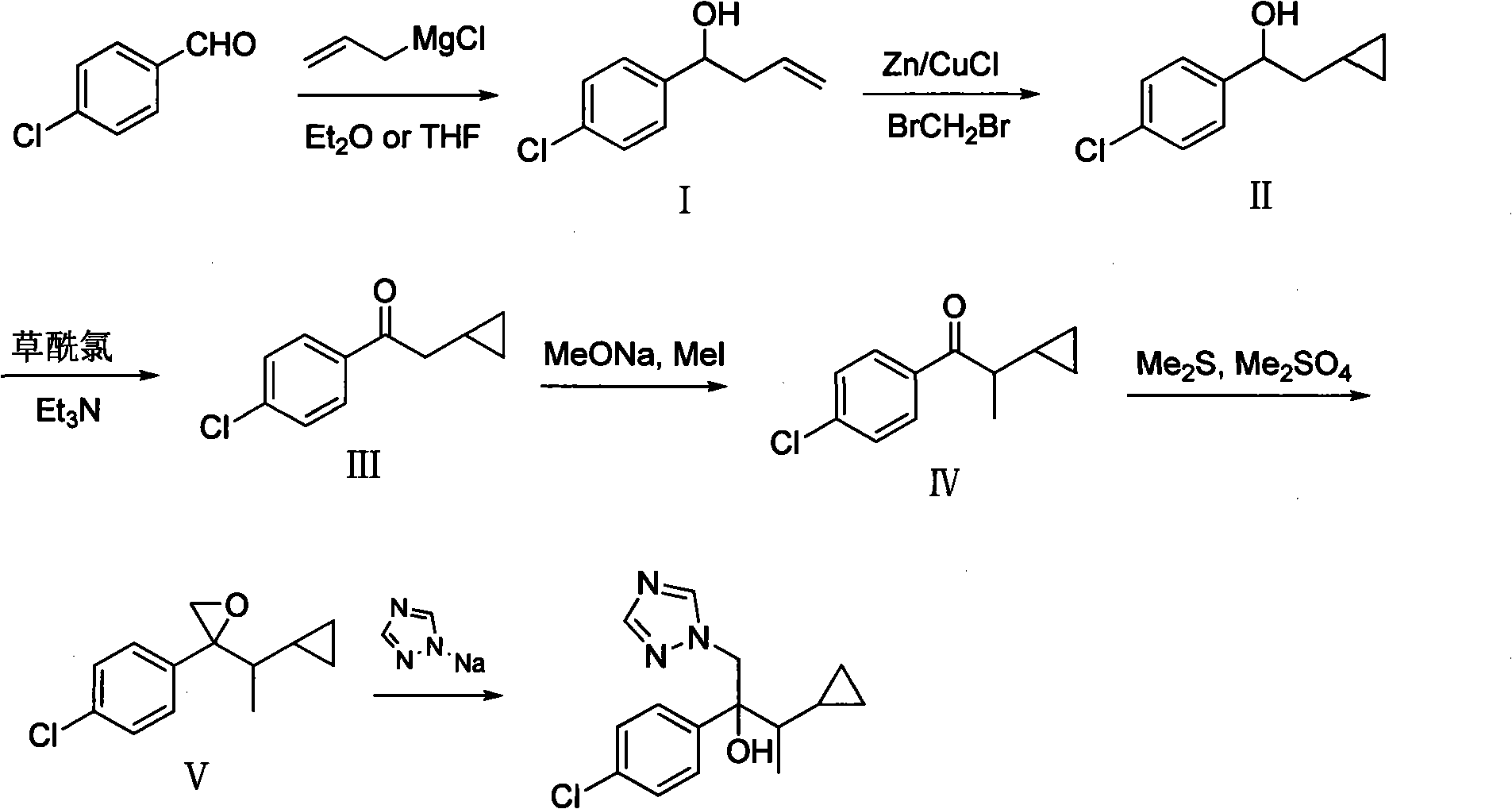
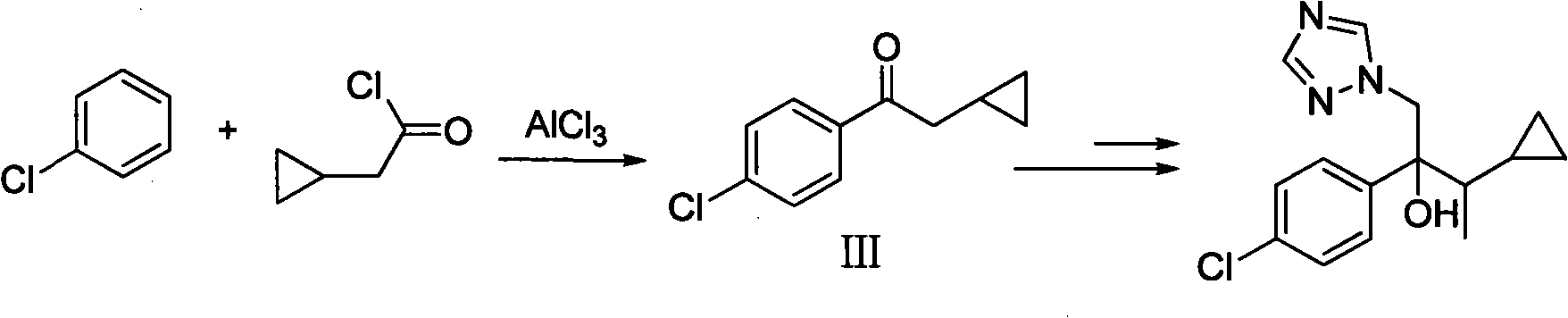
Simple method for preparing cyproconazole by cyclopropyl methyl ketone
A technology of cyclopropyl methyl ketone and cyproconazole, which is applied in the field of easy preparation of cyproconazole through cyclopropyl methyl ketone, and can solve the problems that it is difficult to meet the requirements of green chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The following examples will help to understand the present invention, but can not limit the content of the present invention.
[0028] Synthesis of compound VII:
[0029] Dissolve compound VI (1.6g, 10mmol) in anhydrous ether (8.0mL), add the above solution (2.0mL) to a mixture of magnesium chips and anhydrous ether (264mg, 11.0mmol), heat to initiate the reaction to reflux, Add the ether solution of the remaining compound VI into the system dropwise, keep the system boiling, heat and reflux for 10 min, then cool to room temperature, and set aside.
[0030] Dissolve cyclopropylmethyl ketone (1.87mL, 20mmol) in anhydrous ether (20.0mL), cool in an ice bath to 0°C, slowly add the prepared lattice reagent into the system dropwise, and a white precipitate immediately precipitates out (grid Reagent precipitation). After the dropwise addition, the system was raised to room temperature and stirred for 1 h. The reaction was quenched with saturated ammonium chloride aqueous s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com