Furocoumarin compound with hypertension activity reducing function and preparation method thereof
A technology of furanocoumarins and compounds, which is applied in the field of biomedicine, can solve the problems of toxic and side effects of chemical drugs, and the unforeseen effects of chemical drug treatment, and achieve the effects of cheap and easy-to-obtain reagents, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
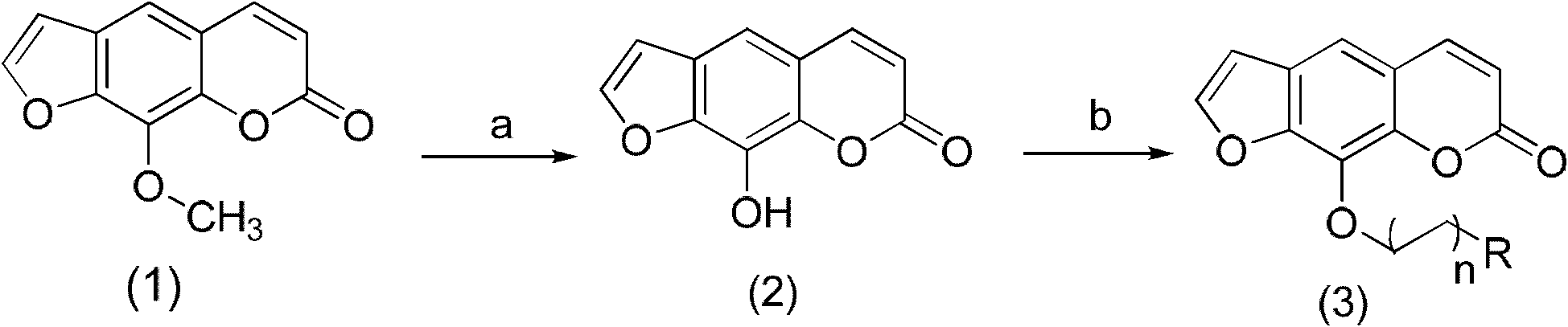
[0038] Example 1 In the structural formula, n is 2 and forms a double bond, and R is a compound of dimethyl, prepared by the following steps:
[0039] 1) Xanthotoxin (1) is demethylated by boron tribromide to obtain the compound Xanthoxylin (2)
[0040] Take 4.32g (20.00mmol) prickly ash toxin in a 250ml eggplant-shaped bottle, add 73ml of anhydrous dichloromethane, shake well to dissolve, put it in an ice bath under nitrogen protection, and stir for 10-15min; at the same time, put Dissolve 6.88ml of boron tribromide in 73ml of anhydrous dichloromethane to prepare a 1mol / L boron tribromide-dichloromethane solution for use.
[0041] When the temperature inside the eggplant-shaped bottle is constant, place the prepared boron tribromide-dichloromethane solution in a constant pressure dropping funnel, slowly drop it into the eggplant-shaped bottle stirred in an ice bath, and finish the dripping within 30 minutes. After the drop, the ice bath was removed, and the reaction was carr...
Embodiment 2
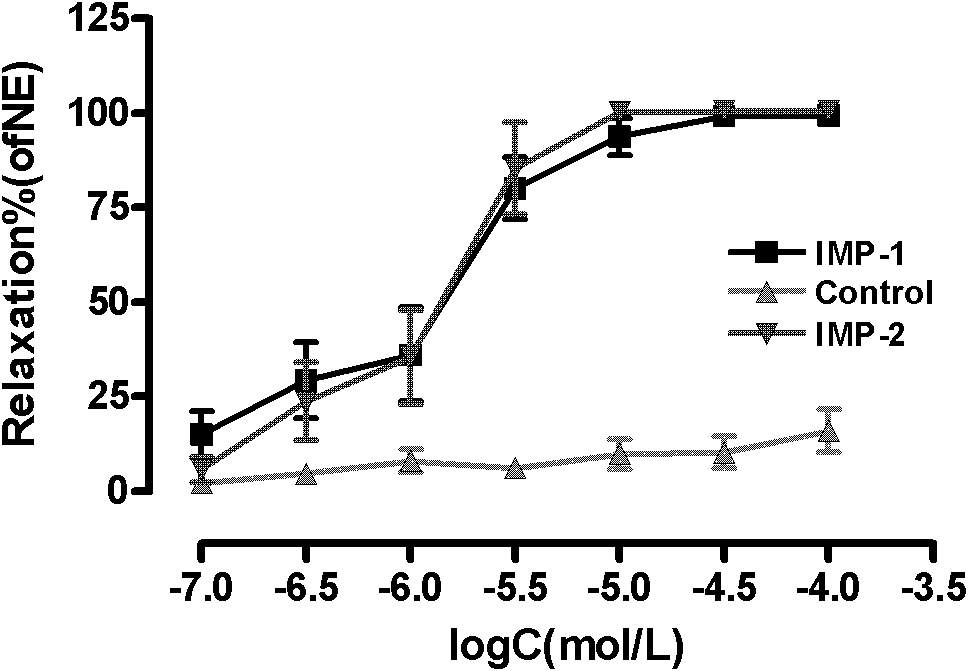
[0049] Example 2 The compound in which n is 2 and R is dimethyl in the structural formula is prepared by the following steps:
[0050] Step 1) is the same as in Example 1, that is, the preparation steps from the compound xanthotoxin (1) to the compound xanthoxylin (2) are the same; after that, the etherification reaction between the phenolic hydroxyl group and isopentyl bromide occurs, specifically:
[0051] Dissolve 0.40g (2.00mmol) of compound (2) in 10ml of treated anhydrous N,N-dimethylformamide (DMF), add 0.83g (6.00mmol) of anhydrous potassium carbonate, stir at room temperature for 30min, and then Add 0.37ml (3.00mmol) of isopentyl bromide, and react in an oil bath at 80°C for 20 hours under nitrogen protection. After the reaction was cooled to room temperature, the whole system was poured into ice water, stood still until the ice melted, extracted several times with ethyl acetate until the ethyl acetate layer was colorless, and after TLC showed that there was no produc...
Embodiment 3
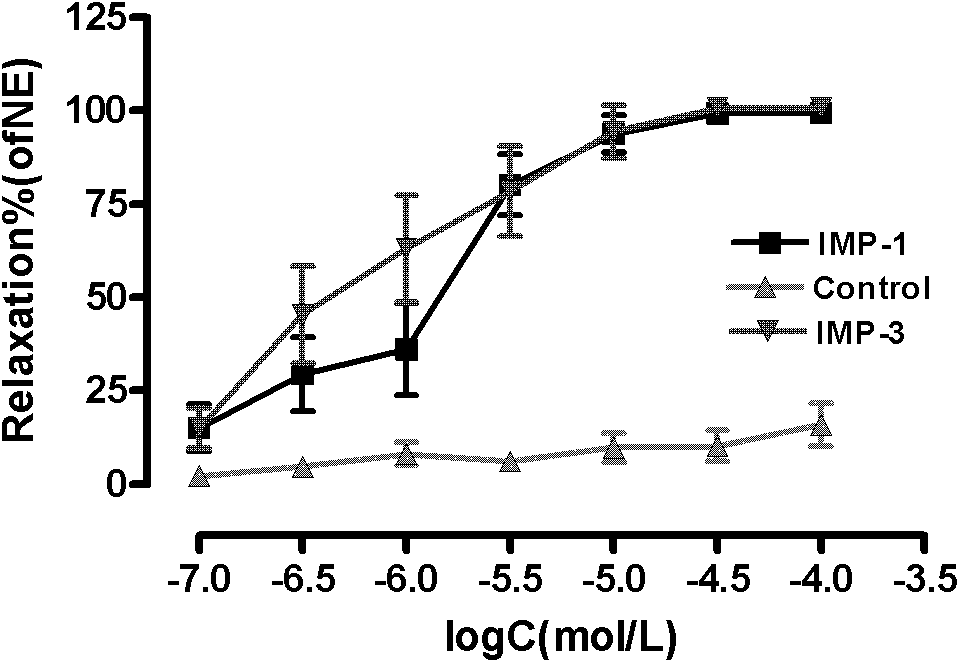
[0055] Example 3 In the structural formula, n is 2 and forms a double bond, and R is a compound of a hydrogen atom, which is prepared by the following steps:
[0056] Step 1) is the same as in Example 1, that is, the preparation steps from the compound xanthotoxin (1) to the compound xanthoxylin (2) are the same; after that, the etherification reaction between the phenolic hydroxyl group and allyl bromide occurs, specifically:
[0057] Dissolve 0.40g (2.00mmol) of compound (2) in 10ml of treated anhydrous N,N-dimethylformamide (DMF), add 0.83g (6.00mmol) of anhydrous potassium carbonate, stir at room temperature for 30min, and then Add 0.25 ml (3.00 mol) of allyl bromide, and react in an oil bath at 80° C. for 19 hours under nitrogen protection. After the reaction was cooled to room temperature, the whole system was poured into ice water, and stood still until the ice melted, and light brown crystals precipitated, filtered with suction, washed with a small amount of water seve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com