Electrode
一种电极、电极基材的技术,应用在电极领域,能够解决损害电极稳定性、钢腐蚀等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0048] According to one embodiment, an electrocatalytic coating, for example comprising a molybdenum component, eg an electrocatalytic oxide coating, is formed on the electrode substrate by means of in situ electrodeposition, ie inside the electrolytic cell.
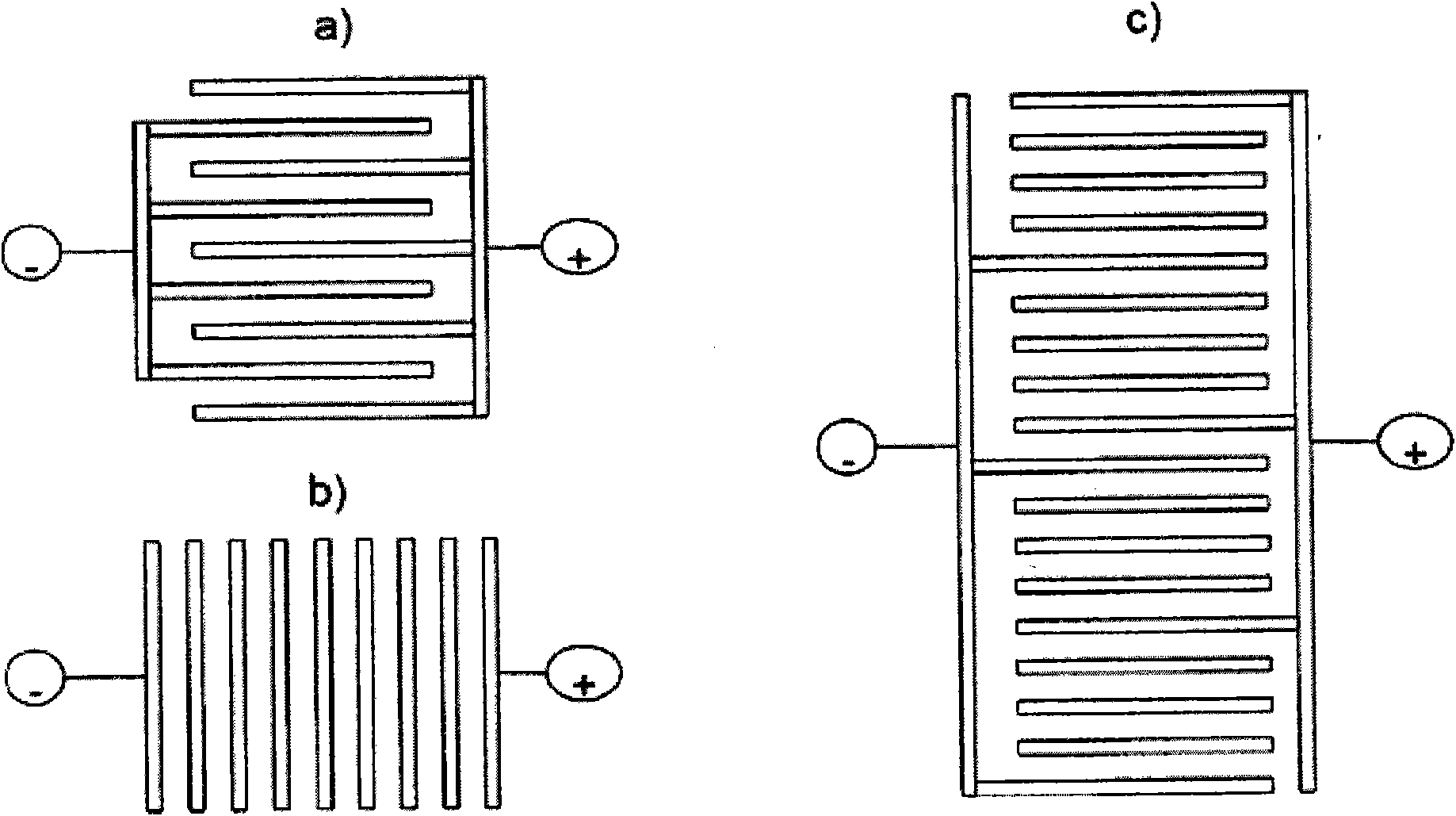
[0049] The invention also relates to an electrode obtainable by the method defined herein. The invention also relates to the use of an electrode as defined herein in an electrolytic cell, for example for the electrolytic production of monochloroacetic acid, for example by reducing dichloroacetic acid or chlorinated acetic acid. However, the electrodes can also be used for arbitrary α-chlorination of carboxylic acids. The invention also relates to an electrode as defined herein for use in an electrolytic flotation process. The electrodes can also be used in several other applications including the production of alkali metal chlorates, HVDC (high voltage direct current) applications, especially in polarity reversal after ...
Embodiment 1
[0075] A small-scale chlorate production trial using an electrolytic cell and a reaction vessel (also used as a gas separator). The electrolyte is circulated by means of a pump. At the top of the reaction vessel, the gas is withdrawn where a small amount of chlorine gas is absorbed in 5 molar sodium hydroxide and water is completely eliminated by adsorption in a desiccant. The oxygen content of the remaining gas is then continuously determined in % by volume. The flow (l / s) of the same gas was also measured to calculate the cathodic current efficiency (CCE) on the cathode. The hydrogen flow rate is determined by subtracting the oxygen portion from the total gas flow rate. The CCE is then calculated from the hydrogen flow rate using the following expression CCE=(standard l / sH 2 / 22.4)·(2F / I), where F is Faraday's constant and I is the current in amperes through the electrolytic cell.
[0076] The starting electrolyte used is containing 120g / L NaCl and 580g / L NaClO 3 of aqu...
Embodiment 2
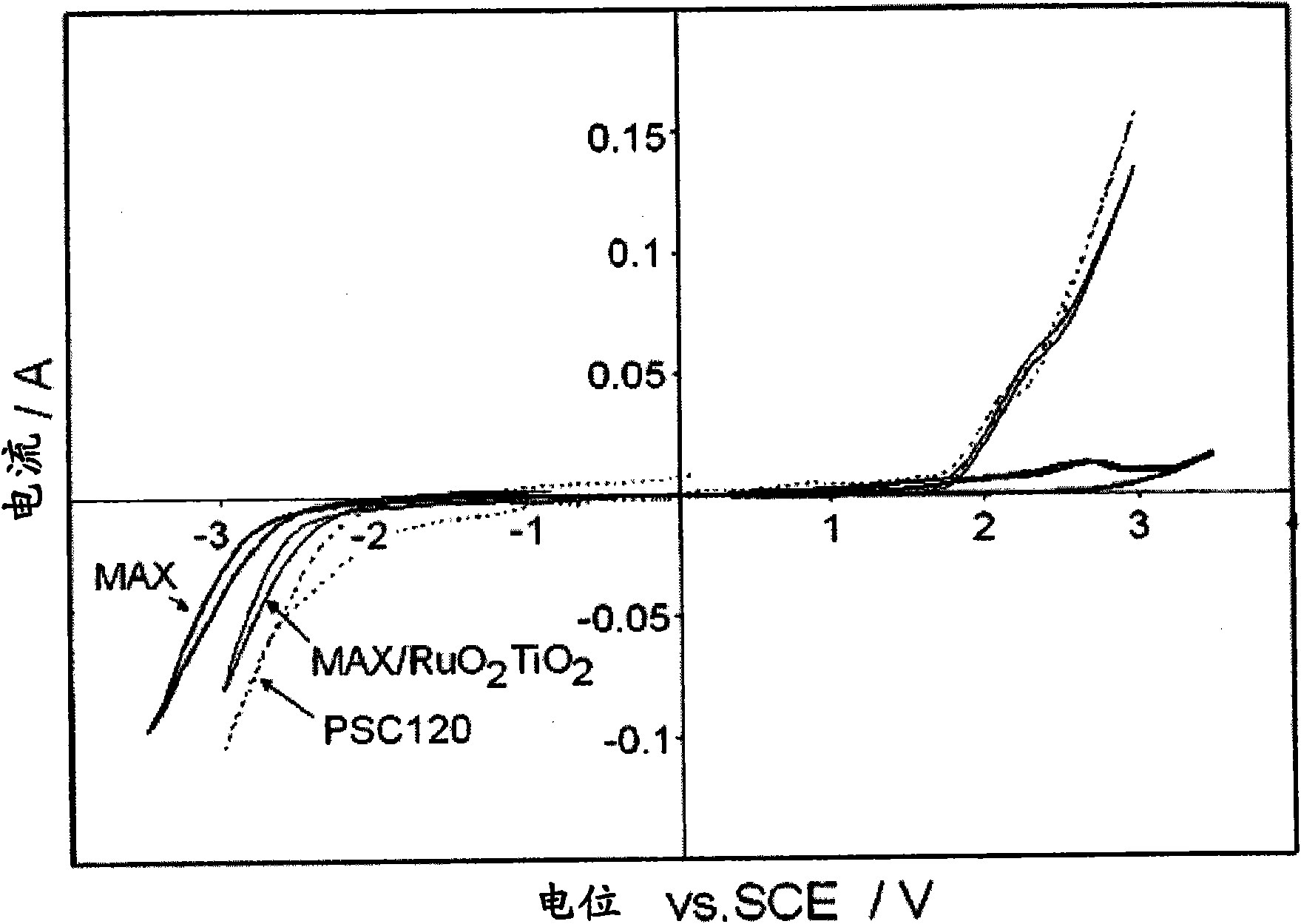
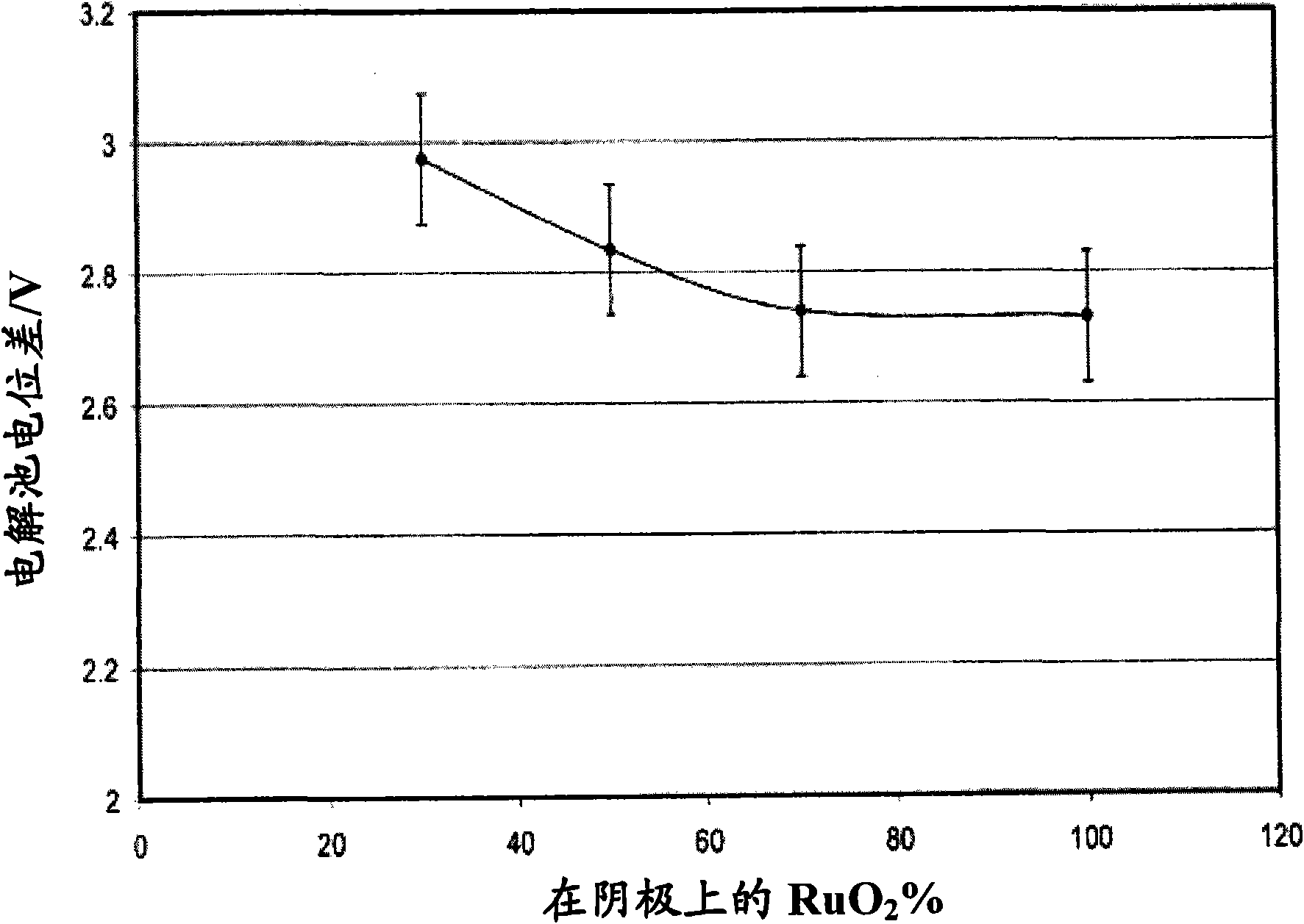
[0082] The same experimental design as in Example 1 was used. The initial electrolyte used is containing 120g / LNaCl, 580g / L NaClO 3 and 4.4g / L Na 2 Cr 2 o 7 of aqueous solution. The anode in the electrolytic cell is PSC120 (DSA ,TiO 2 / RuO 2 ), available from Permascand. Cathode material is MAXTHAL with machined surface 312 (4.1g / cm 3 ). The distance between the anode and cathode is about 4mm. The exposed surface area is 30cm 2 . 3kA / m was used throughout the experiment 2 the current density. The results are presented in Table 2.
[0083] Table 2
[0084]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com