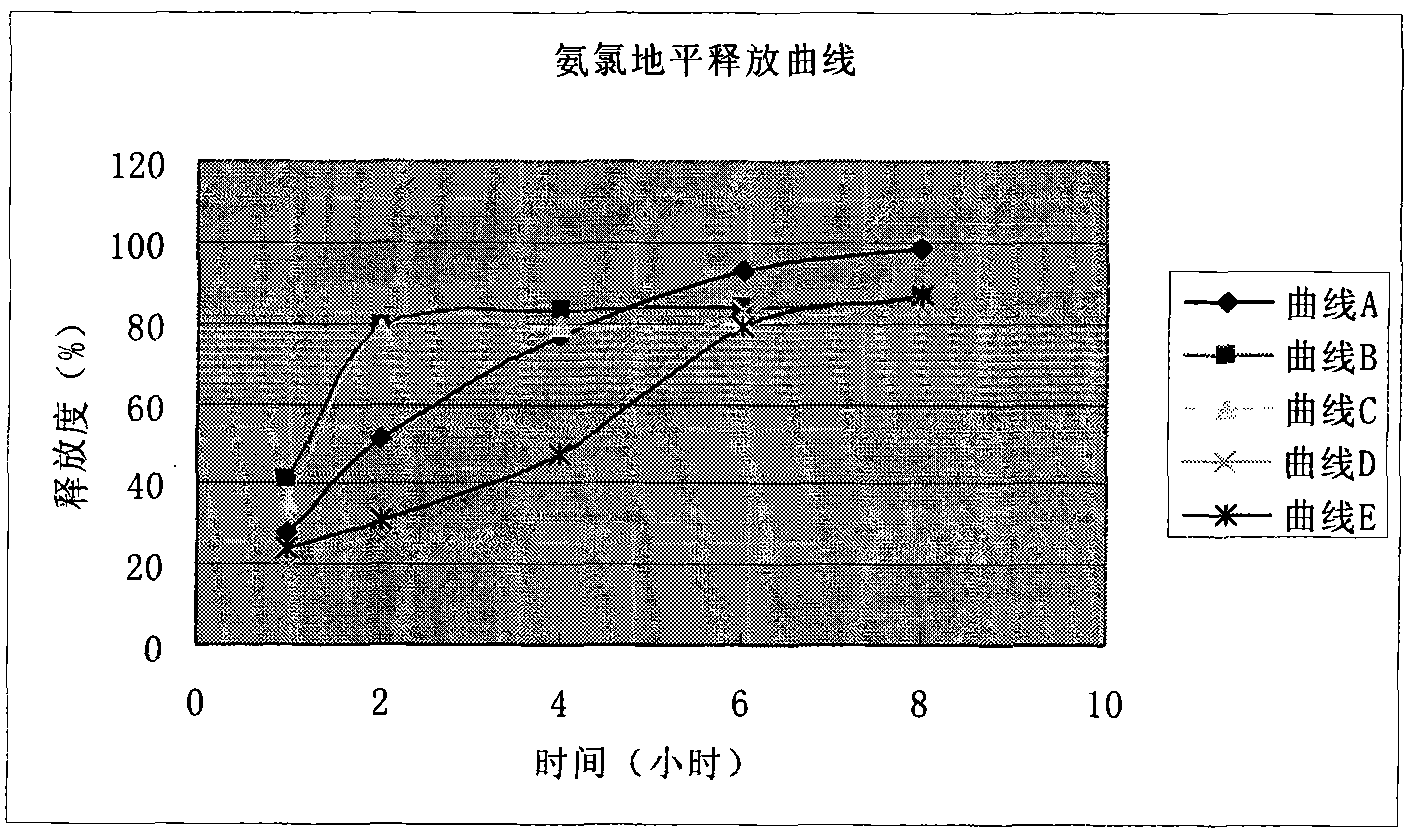
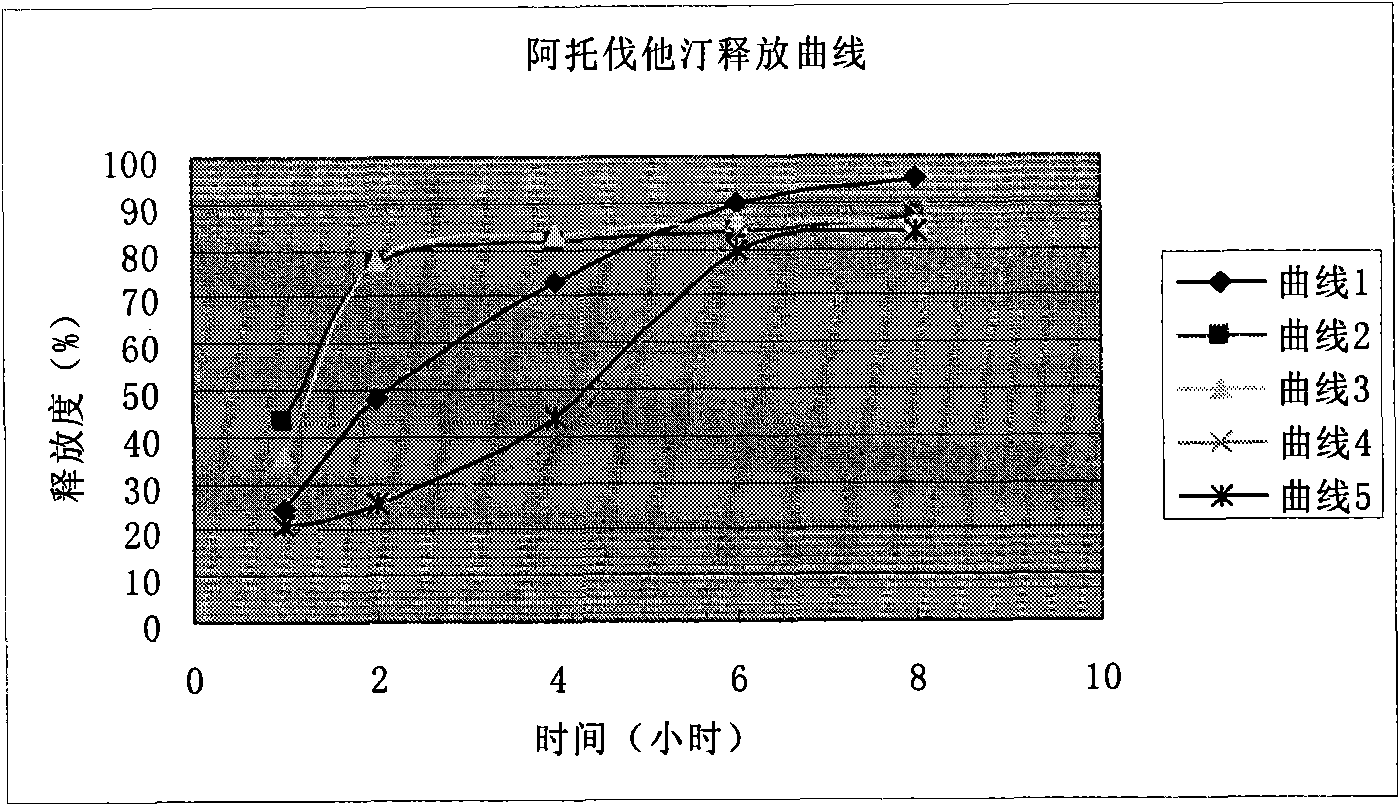
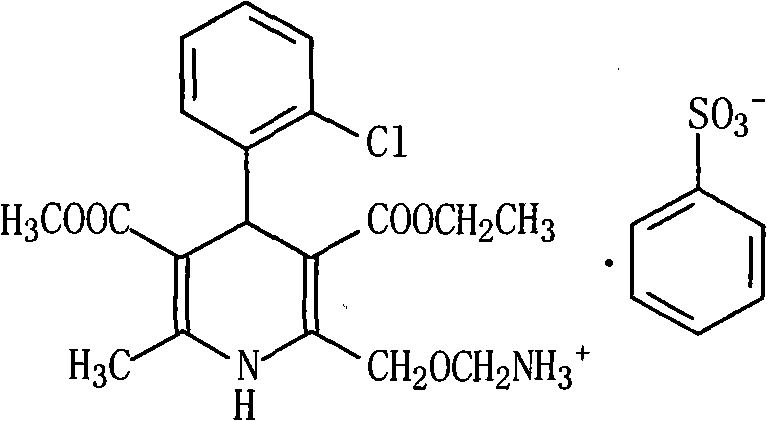
Amlodipine besylate and atorvastatin calcium medicine compound liposome solid preparation
A technology of atorvastatin calcium and atorvastatin, which is applied in the direction of drug combination, liposome delivery, active ingredients of heterocyclic compounds, etc., and can solve the problems of single drug and inability to fully control mild to moderate hypertension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation prescription (1000) of embodiment 1 amlodipine atorvastatin calcium liposome tablet:
[0072] Amlodipine besylate (calculated as amlodipine) 5g
[0073] Atorvastatin calcium (calculated as atorvastatin) 10g
[0074] Soy Phosphatidylglycerol 20g
[0075] Sodium Glycocholate 4g
[0076] Cholesterol 8g
[0077] Starch 38g
[0078] Lactose 30g
[0079] Crospovidone 6g
[0080] Povidone K30 5g
[0081] Magnesium Stearate 1.5g
[0082] Preparation Process:
[0083] (1) Dissolve 20g of soybean phosphatidylglycerol, 4g of sodium glycocholate and 8g of cholesterol in 400ml of a mixed solvent of dichloromethane and ether at a volume ratio of 1:5, mix well, and decompress on a rotary film evaporator Remove the mixed solvent to obtain a phospholipid film;
[0084] (2) Add phosphoric acid-disodium hydrogen phosphate buffer solution with a pH value of 6.0, shake and stir to completely hydrate the phospholipid membrane, emulsify at a high speed, and filter th...
Embodiment 2
[0089] The preparation prescription (1000) of embodiment 2 amlodipine atorvastatin calcium liposome tablet:
[0090]Amlodipine besylate (calculated as amlodipine) 5g
[0091] Atorvastatin calcium (calculated as atorvastatin) 80g
[0092] Soy Phosphatidylglycerol 120g
[0093] Sodium Glycocholate 30g
[0094] Cholesterol 40g
[0095] Microcrystalline Cellulose 55g
[0096] Lactose 76g
[0097] Low-substituted hydroxypropyl cellulose 25g
[0098] Hypromellose 4g
[0101] Preparation Process:
[0102] (1) Dissolve 120g of soybean phosphatidylglycerol, 30g of sodium glycocholate and 40g of cholesterol in 700ml of a mixed solvent of dichloromethane and ether at a volume ratio of 1:5, mix well, and decompress on a rotary film evaporator Remove the mixed solvent to obtain a phospholipid film;
[0103] (2) Add phosphoric acid-disodium hydrogen phosphate buffer solution with a pH value of 6.0, shake and stir to complete...
Embodiment 3
[0109] The preparation prescription (1000) of embodiment 3 amlodipine atorvastatin calcium liposome capsules:
[0110] Amlodipine besylate (calculated as amlodipine) 10g
[0111] Atorvastatin calcium (calculated as atorvastatin) 10g
[0112] Soy Phosphatidylglycerol 150g
[0113] Sodium Glycocholate 30g
[0114] Cholesterol 50g
[0115] Starch 90g
[0116] Carboxymethyl Starch Sodium 15g
[0117] Povidone K30 10g
[0119] Preparation Process:
[0120] (1) Dissolve 150g of soybean phosphatidylglycerol, 30g of sodium glycocholate and 50g of cholesterol in 800ml of a mixed solvent of dichloromethane and ether at a volume ratio of 1:5, mix well, and decompress on a rotary film evaporator Remove the mixed solvent to obtain a phospholipid film;
[0121] (2) Add citric acid-sodium citrate buffer solution with a pH value of 6.0, shake and stir to completely hydrate the phospholipid membrane, emulsify at a high speed, and filter through a 0.45 μm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com