Application of 5-aminolevulinic acid in preparing medicaments for treating HPV (Human Papilloma Virus) infection
A technology of aminolevulinic acid and HPV virus, applied in antiviral agents, drug combinations, diseases, etc., can solve the problems of long treatment cycle, unsatisfactory curative effect, vaginal mucosa damage, etc., and achieve the effect of fast drug metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Hydrochloride-5-Aminolevulinic Acid Treatment of Persistent Infection, Infection with Viral Load ≥ 10
[0026] Patient selection method: The patients were detected by the HC2 second-generation hybridization capture technology, and the second detection was performed at least one year later. During the two detection periods, the patients did not receive photodynamic therapy with 5-aminolevulinic acid hydrochloride. Those who were positive twice were selected.
[0027] Medication method: use 500mg / hypohydrochloride-5-aminolevulinic acid powder, pour the powder into the temperature-sensitive gel (2ml), stir gently to make it evenly mixed, or dissolve it in sterile aqueous solution to prepare a solution with a concentration of 23%. agent. Clinical treatment of 86 patients with high-risk HPV infection, all patients had HPV infection for more than 1 year, and the viral load was greater than 10.
[0028] Apply the gel or solution evenly on the patient's vagina and c...
Embodiment 2
[0030] Example 2 Interferon treatment of persistent infection, the method of HPV infection with viral load ≥ 10
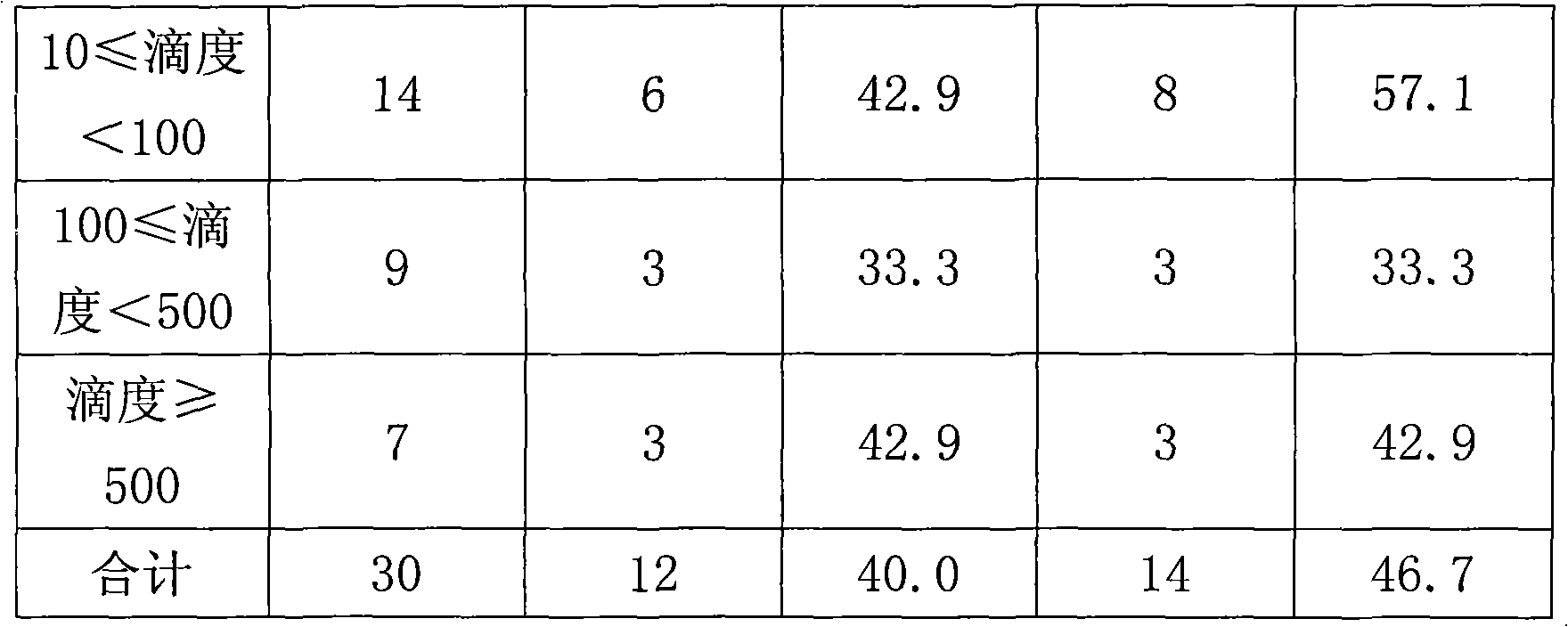
[0031] Another 30 patients with high-risk HPV infection were treated with interferon as a control. All patients were selected in the same way as above, and all of them had HPV infection for more than 1 year, and their viral load was ≥10. The treatment plan is: recombinant interferon α-2a suppository, 6×10 4 IU / capsule, non-menstrual vaginal administration, place the suppository in the posterior fornix of the vagina, 1 capsule / time, once every other day. 6 times as a course of treatment, 2 consecutive courses of treatment. Follow-up and HPV detection were performed 1 month and 3 months after the end of treatment.
Embodiment 3
[0032] Example 3 Follow-up results of hydrochloride-5-aminolevulinic acid and interferon in the treatment of persistently infected HPV
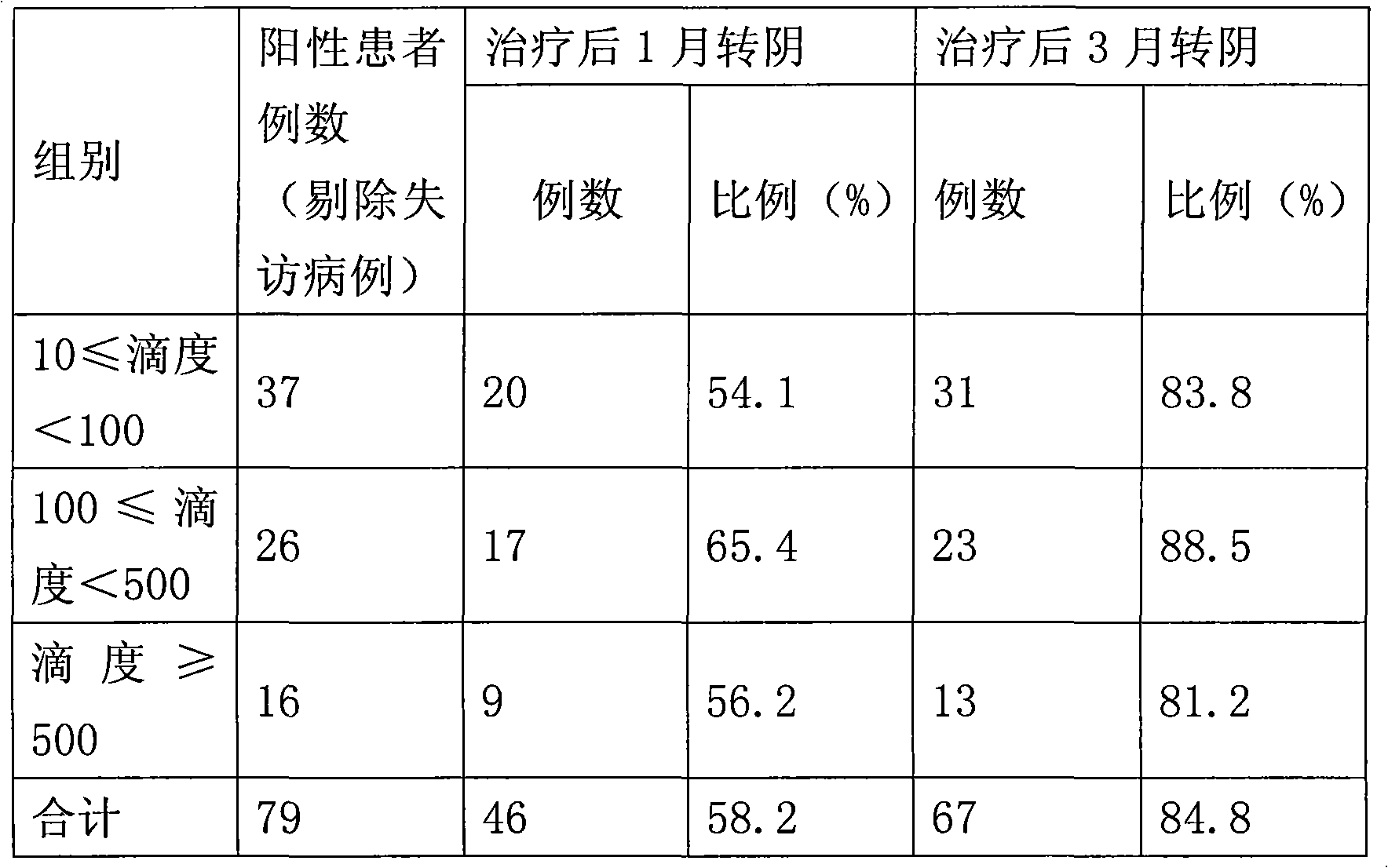
[0033] 1), 5-aminolevulinic acid group: a total of 86 patients, 7 cases dropped out, and 79 cases remained. During the first follow-up visit, 46 patients had their HPV virus detection changed from positive to negative (viral titer was lower than 1), accounting for 58.2%, and 71 patients had their HPV virus detection value dropped below 10, accounting for 89.87%; the remaining 8 The detection value of HPV in 1 patient decreased, but the decrease was not obvious. During the second follow-up visit, 67 patients had negative virus tests, accounting for 84.8%, and 77 patients had virus titers lower than 10, accounting for 97.5%. The number of patients who relapsed after one year of follow-up observation and turned negative was zero.
[0034] Comparison of the effect of 5-aminolevulinic acid hydrochloride on the titer of different viruses after on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com