Method for preparing cationic polymer for increasing and/or stabilizing viscosity of hydrophobic association type polymer oil-displacing agent
A technology of cationic polymers and degree of polymerization, which is applied in the direction of chemical instruments and methods, drilling compositions, etc., can solve the problems of enhancing hydrophobic association, accelerating the rate of association, intensifying association, etc., and achieving polymerization conversion rate High, improve the use of efficiency, high viscosity stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of cationic polymer
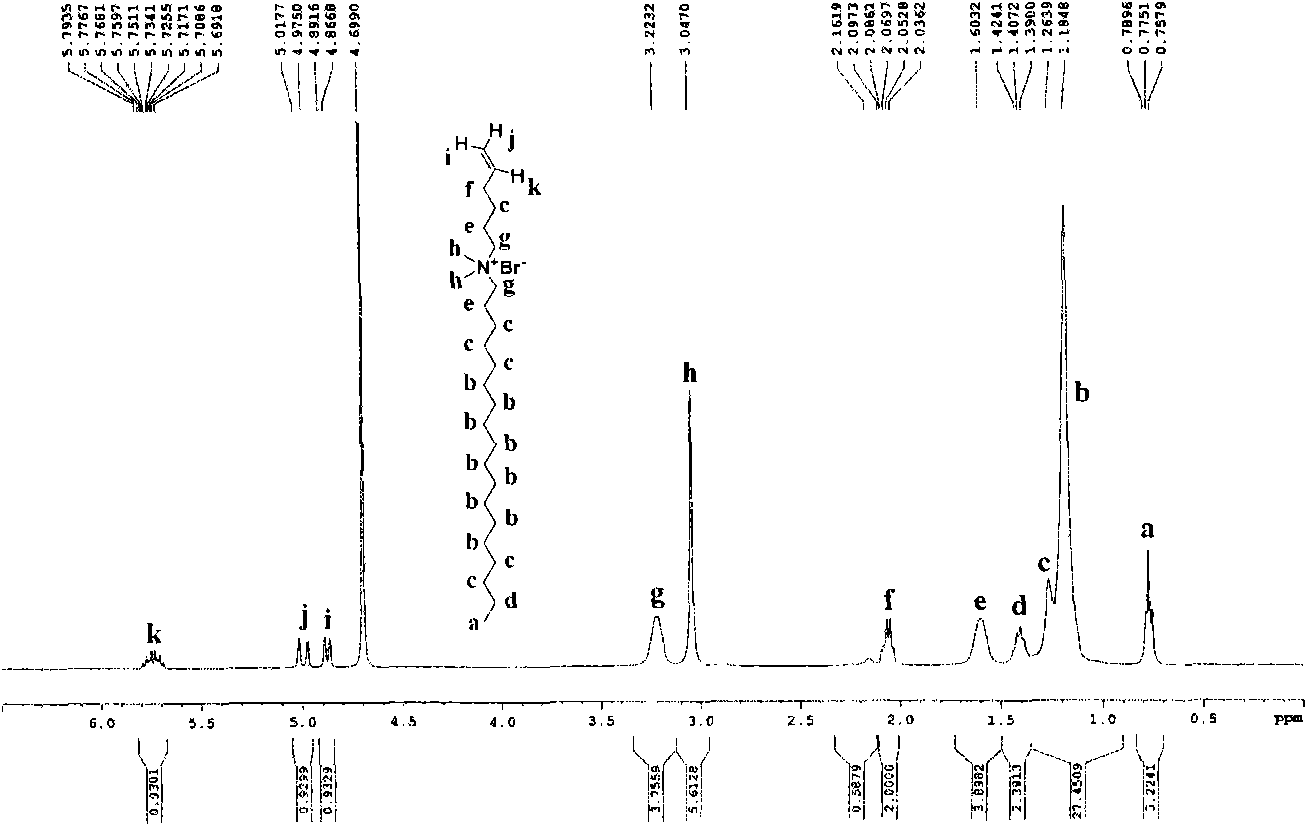
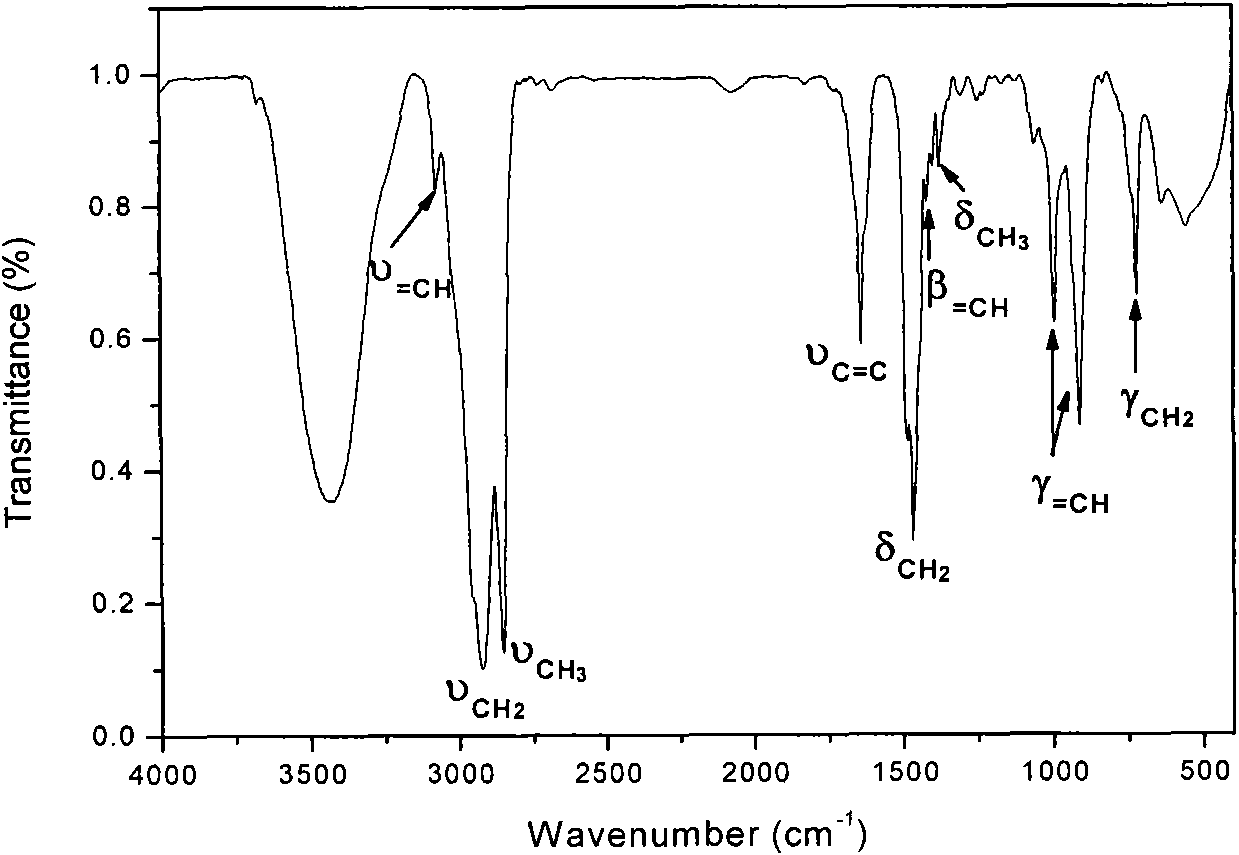
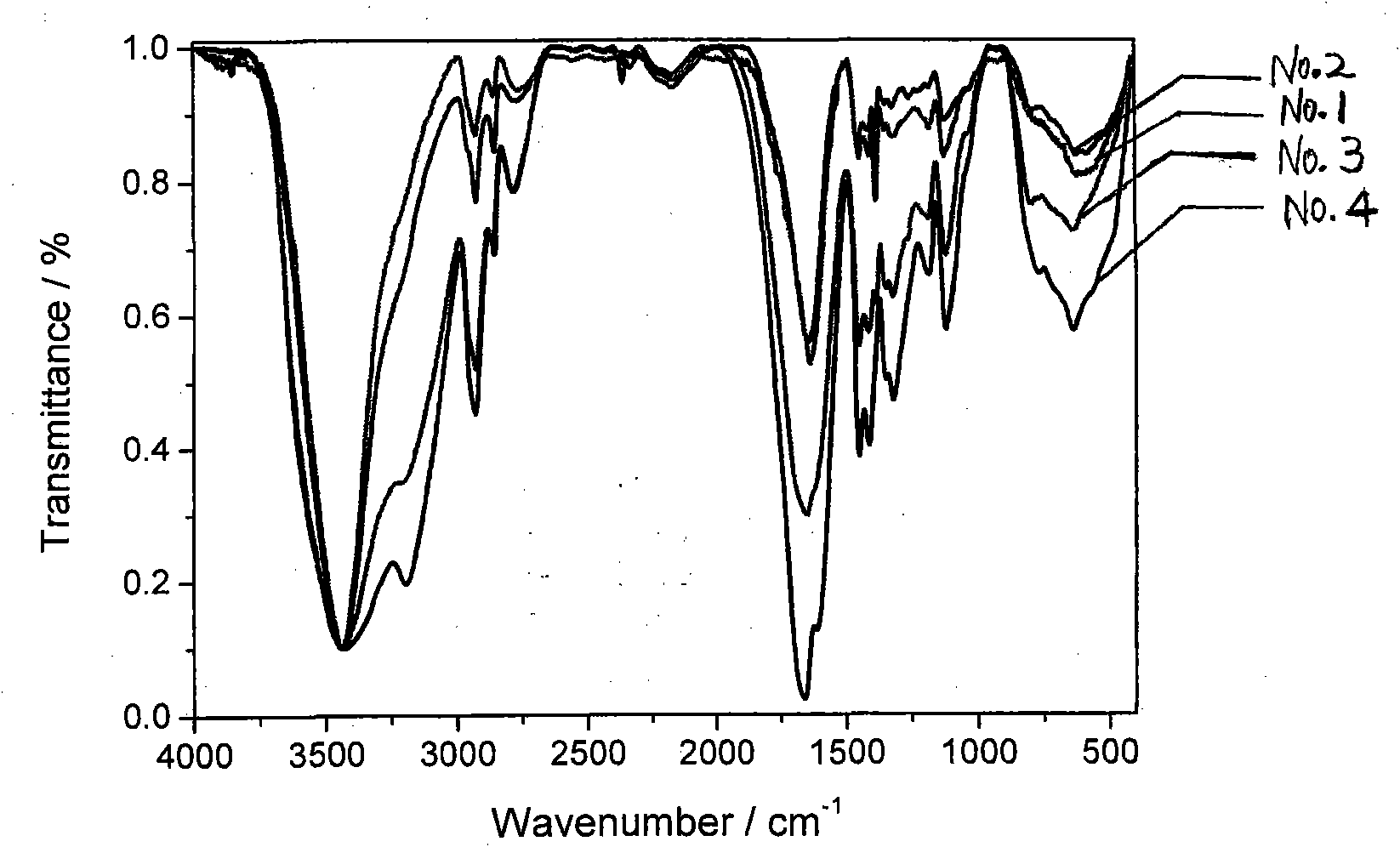
[0043] Add acrylamide (Sinopharm Chemical Reagent Co., Ltd.), cetyldimethyl-5-enhexyl ammonium bromide, sodium lauryl sulfate (Tianjin Kemeng Chemical Industry and Trade Co., Ltd.), and deionized water In the reactor, feed nitrogen to stir, place the reaction vessel in a constant temperature water bath at 80°C, add potassium persulfate (Sinopharm Chemical Reagent Co., Ltd.) The product is dried in a vacuum oven to prepare a cationic polymer. Wherein the mol ratio of acrylamide, hexadecyldimethyl-5-enhexyl ammonium bromide is 98: 2, sodium lauryl sulfate accounts for 32% of the total mass of the reaction monomer, and the ratio of reactant and deionized water The mass ratio is 12:88, and the addition amount of the initiator is 0.20% of the reaction monomer. The product is represented by No.1, the yield is 80.23%, and the cationic degree is 0.025%.
[0044] The structural formula of this cationic polymer is formula (I)...
Embodiment 2
[0047] Embodiment 2, the preparation of cationic polymer
[0048] Add acrylamide, hexadecyldimethyl-5-enhexylammonium bromide, sodium lauryl sulfate, and deionized water into the reactor, blow in nitrogen gas and stir, and place the reaction vessel in a constant temperature water bath at 75°C , and then add potassium persulfate initiator, continue nitrogen reaction for 6 hours, place the obtained milky white colloidal product in a vacuum drying oven to dry, and prepare a cationic polymer. Wherein the mol ratio of acrylamide, hexadecyldimethyl-5-enhexyl ammonium bromide is 98: 2, sodium lauryl sulfate accounts for 47% of the total mass of the reaction monomer, and the ratio of reactant and deionized water The mass ratio is 12:88, and the addition amount of the initiator is 0.18% of the reaction monomer. The product is represented by No.2, the yield is 88.57%, and the cationic degree is 0.29%.
[0049] The structural formula of this cationic polymer is formula (I), and in the ...
Embodiment 3
[0052] Embodiment 3, the preparation of cationic polymer
[0053] Add acrylamide, hexadecyldimethyl-5-enhexylammonium bromide, sodium lauryl sulfate, and deionized water into the reactor, blow in nitrogen gas and stir, and place the reaction vessel in a constant temperature water bath at 70°C , and then add potassium persulfate initiator, continue nitrogen reaction for 6 hours, place the obtained milky white colloidal product in a vacuum drying oven to dry, and prepare a cationic polymer. Wherein the mol ratio of acrylamide, hexadecyldimethyl-5-enhexyl ammonium bromide is 98: 2, sodium lauryl sulfate accounts for 18% of the total mass of the reaction monomer, and the ratio of reactant to deionized water The mass ratio is 9:91, and the addition amount of the initiator is 0.17% of the reaction monomer. The product is represented by No.3, the yield is 85.85%, and the cationic degree is 0.66%.
[0054] The structural formula of this cationic polymer is formula (I), and in the fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com