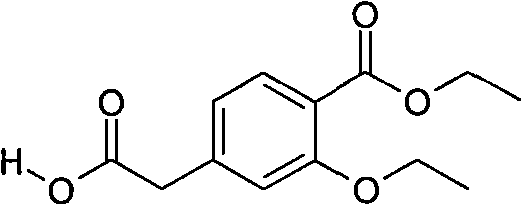
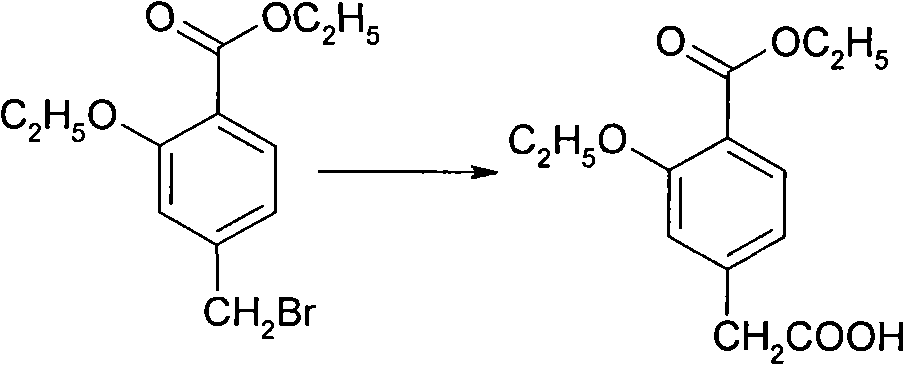
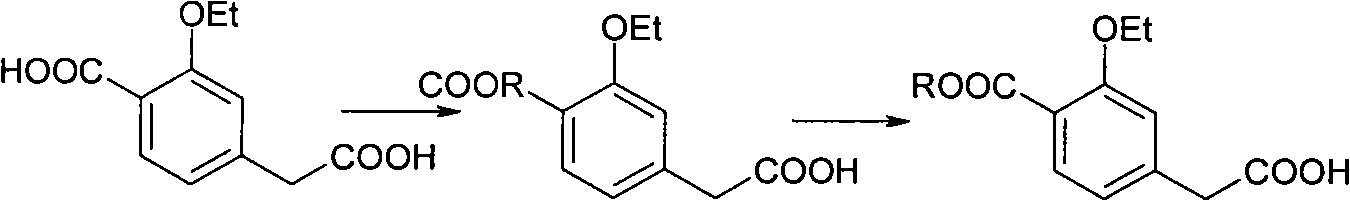
Compounding method for 3- ethyoxyl-4-ethoxycarbonyl phenylacetic acid
A technology of ethoxycarbonyl benzene and a synthesis method, which is applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., can solve the problems of complicated steps, large environmental pollution, many side reactions, etc., and achieves simple industrial operation. , Environmentally friendly and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] 1) Alkylation: In a dry 1000-liter reaction pot, vacuum pump 300 kilograms of N,N-dimethylformamide, add 200 kilograms of potassium carbonate and 100 kilograms of 4-methylsalicylic acid under stirring, control Add 200 kg of bromoethane dropwise at 0°C, and keep the temperature at 10°C for 24 hours after dropping. 200 kg of toluene was added and stirred for 30 minutes, and the white solid was removed by suction filtration. The filtrate was washed with 300 kg of water and separated into layers. The organic phase was dried with 30 kg of anhydrous magnesium sulfate for 24 hours, filtered with suction, and the filtrate was concentrated to dryness to obtain 135 kg of oily ethyl 2-ethoxy-4-methylbenzoate with a GC purity of 99.8%.
[0029] 2) Acylation: 900 kg of 2-methyltetrahydrofuran and 200 kg of diisopropylamine were sucked into a dry stainless steel reaction pot of 2000 liters by vacuum. The jacket was fed with liquid nitrogen, and the temperature was lowered to -40°C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com