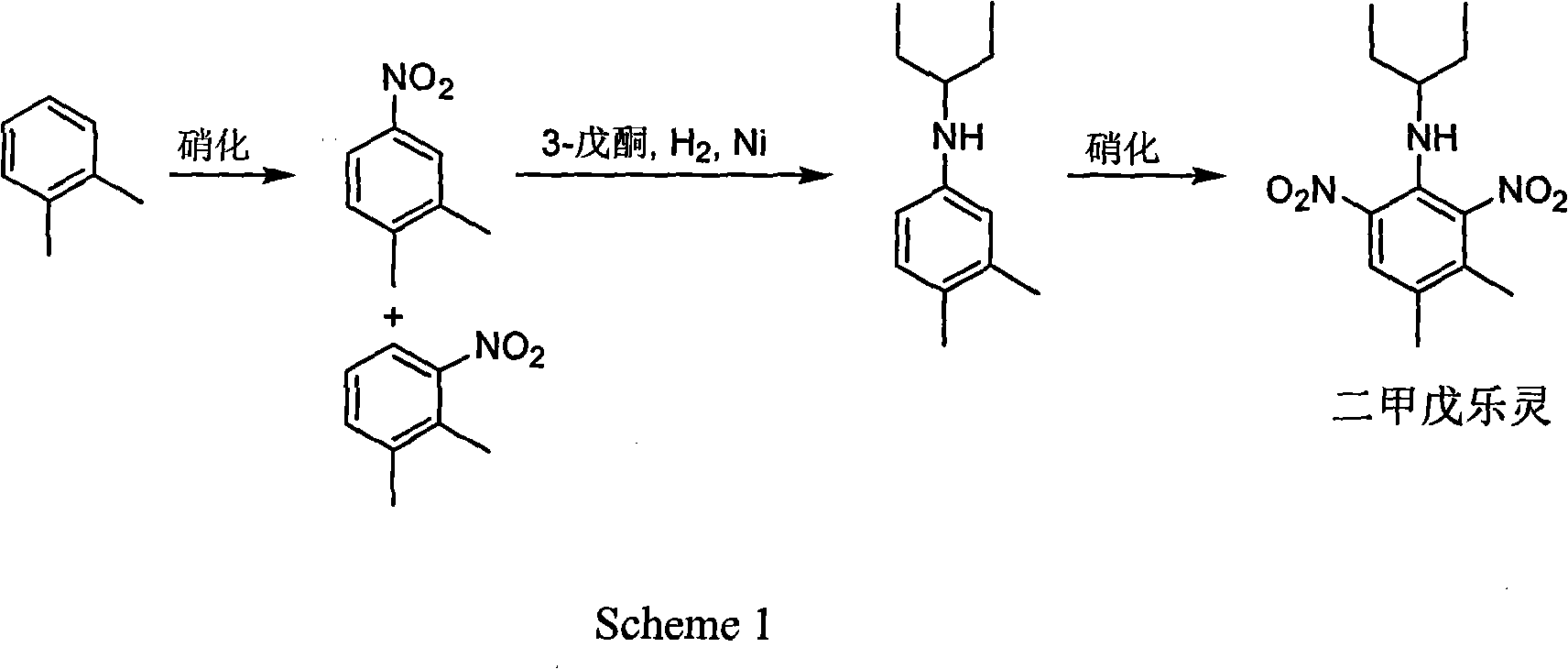
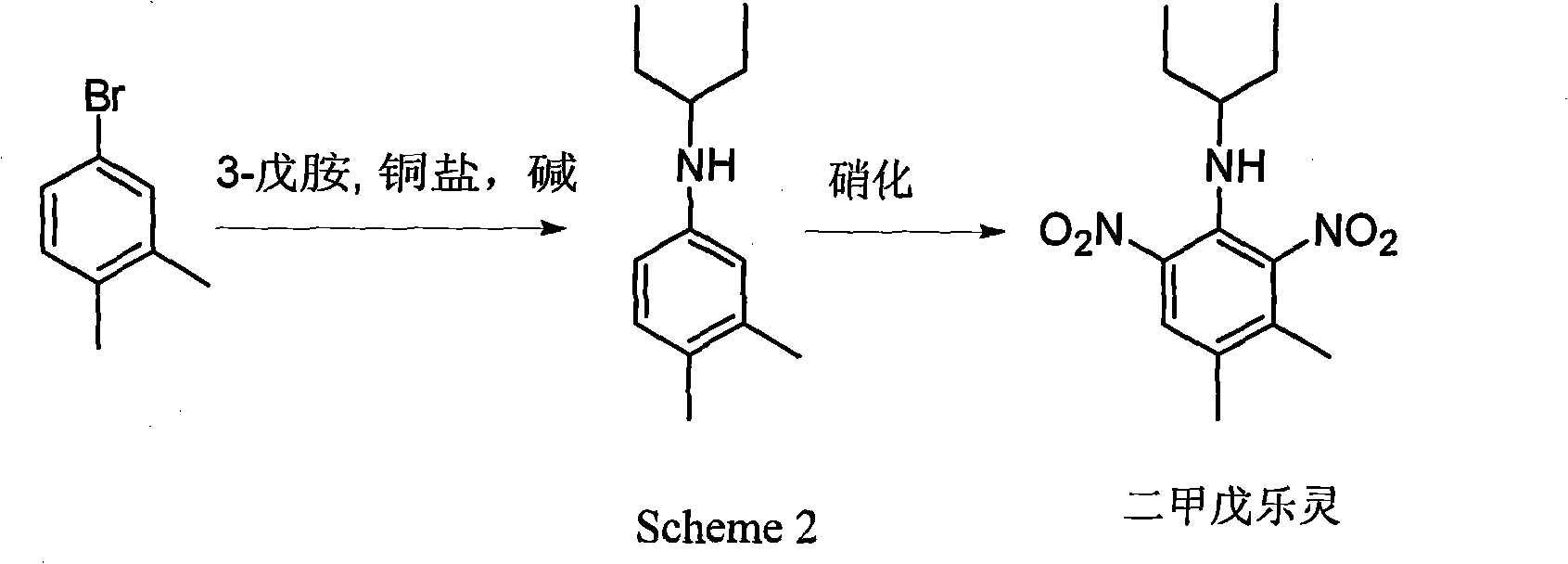
Method for preparing pendimethalin
A pendimethalin and dimethyl technology, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of long routes and achieve simple equipment requirements, high equipment utilization, and easy The effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Under nitrogen protection, 15 ml of anhydrous DMF, 3.72 g of m-bromo-o-xylene, 3.48 g of 3-pentylamine, 8.48 g of anhydrous potassium phosphate, 0.19 g of cuprous iodide and 0.76 g of N, N-diethyl salicylamide, the above reaction mixture was reacted at 80° C. for 24 hours under stirring. Cool to room temperature, add 30 ml of water, extract with ethyl acetate (15 ml x 2 times), separate the organic phase, distill off the solvent ethyl acetate, and the remaining 3.55 g of crude product is directly used for the next step of nitration reaction.
[0023] The operation method of nitration reaction mainly refers to patents IN 181756 and US5922913. The above 3.55 g of crude product and 6 g of acetic acid were mixed, heated to 60° C., a mixture of 3.2 g of concentrated nitric acid and 3.2 g of acetic acid was added dropwise with stirring, and stirring was continued at 60° C. for 2.5 hours. Cool to room temperature, dilute with 20 mL of water, and extract the product with 1,2-d...
Embodiment 2
[0025] Under nitrogen protection, 15 ml of anhydrous DMF, 3.72 g of m-bromo-o-xylene, 3.48 g of 3-pentylamine, 2.76 g of anhydrous potassium carbonate, 0.19 g of cuprous iodide and 0.76 g of N, N-diethyl salicylamide, the above reaction mixture was reacted at 90° C. for 24 hours under stirring. Cool to room temperature, add 30 ml of water, and extract with toluene (15 ml x 3 times). The organic phase was washed with 15 milliliters of 5% dilute hydrochloric acid, and the aqueous phase was adjusted to pH 8-9 with 30% sodium hydroxide solution, then extracted 2 times with toluene, and the solvent toluene was evaporated, and the remaining crude product (3.32 g) and 6 g Acetic acid was mixed, heated to 60°C, a mixture of 3.2 g of concentrated nitric acid and 3.2 g of acetic acid was added dropwise with stirring, and stirring was continued at 60°C for 2.5 hours. Cool to room temperature, dilute with 20 mL of water, and extract the product with 1,2-dichloroethane (10 mL×3). The lay...
Embodiment 3
[0027] Under nitrogen protection, 20 milliliters of anhydrous DMF, 3.72 grams of m-bromo-o-xylene, 3.48 grams of 3-pentylamine, 2.76 grams of anhydrous potassium carbonate, and 0.29 grams of cuprous oxide were successively added to a 100 milliliter autoclave, and the above reaction mixture was Under stirring, react at 120° C. for 24 hours. Cool to room temperature, add 30 ml of water, and extract with toluene (15 ml x 3 times). The organic phase was washed with 15 milliliters of 5% dilute hydrochloric acid, and the aqueous phase was adjusted to pH 8-9 with 30% sodium hydroxide solution, then extracted twice with ethyl acetate, the solvent ethyl acetate was evaporated, and the remaining crude product (2.96 g ) and acetic acid (6 g), heated to 60°C, a mixture of concentrated nitric acid (3.2 g) and acetic acid (3.2 g) was added dropwise with stirring, and stirring was continued at 60°C for 2.5 hours. Cool to room temperature, dilute with 20 mL of water, and extract the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com