Sulbenicillin sodium compound and new preparation method thereof
A technology of sulfobenicillin sodium and compound, which is applied in the field of medicine and can solve the problems of complicated operation, complicated sulfonation reaction of phenylacetic acid, low product purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
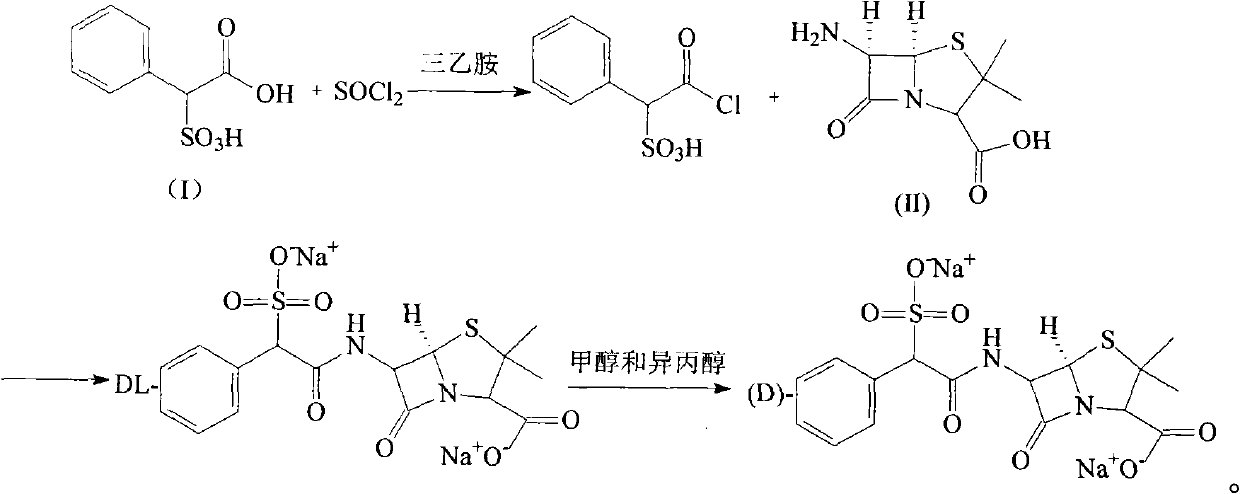
[0026] The synthesis of embodiment 1α-sulfophenylacetyl chloride
[0027] Put 216g (1mol) of α-sulfophenylacetic acid into 400ml of anhydrous diethyl ether, stir well, slowly add 357g (3mol) of thionyl chloride dropwise, after the dropwise completion, add 20ml of triethylamine, at 40°C Stir the reaction for 20 hours, then evaporate diethyl ether and excess thionyl chloride under reduced pressure below 40°C, add 200ml of ether to the residue, evaporate the ether after fully stirring, then add 1000ml of petroleum ether, shake fully, and store at -25°C Freeze for 24 hours, pour off the supernatant, and add 1000ml of dry dichloromethane to the residue to dissolve.
Embodiment 2D
[0028] The synthesis of embodiment 2DL-sulfabenicillin sodium
[0029] Dissolve 216g (1mol) of 6-APA in 2000ml of dichloromethane, slowly add 290ml of triethylamine dropwise under vigorous stirring, then vigorously stir at about 10°C until the solids are completely dissolved, then drop slowly below 0°C Add the dichloromethane solution of α-sulfophenylacetyl chloride obtained in the step, and maintain the pH=7.5 of the reaction with triethylamine at the same time. After dropping, stir and react at room temperature for 2 hours, and pour the reaction solution into 2500ml of ice-water mixture , stirred, allowed to stand for layering, decolorized the water layer with activated carbon, then added 2500ml of n-butanol, adjusted the pH of the reaction solution to 1.5 with 5% sulfuric acid under stirring, collected the organic phase, and used 1000ml of n-butanol for the water layer Alcohol extraction, combined organic phases, and then adding saturated aqueous sodium carbonate to adjust ...
Embodiment 3
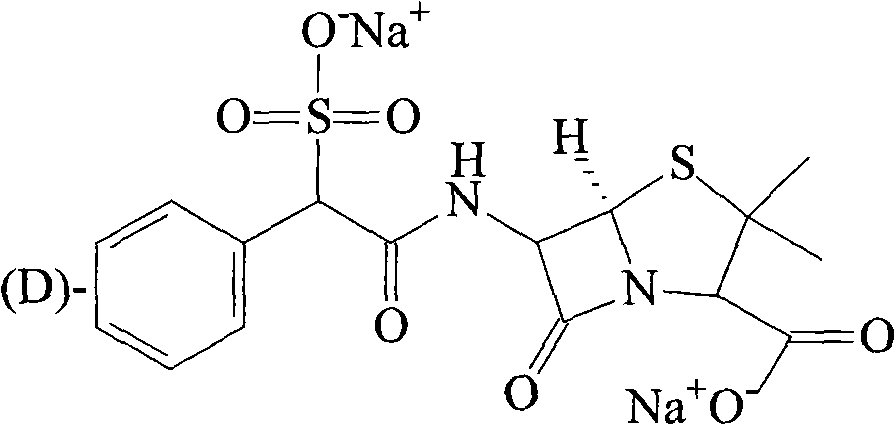
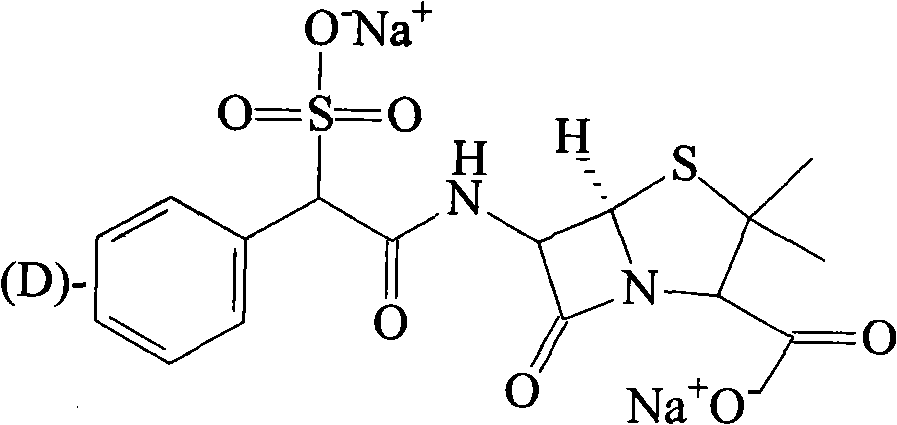
[0030] The synthesis of embodiment 3 sulbenicillin sodium
[0031] 300g of DL-sulfbenicillin sodium was added to the reaction flask, then 1500ml of methanol and isopropanol mixed solvent (1:3), heated to reflux for 2 hours, then cooled to room temperature, stirred for 3 hours, and filtered to obtain a white 105 g of solid (D-sulbenicillin sodium: L-sulbenicillin sodium = 3:1), yield 70%.
[0032] [α] 20 : +179°~+181°.
[0033] IR(KBr)ν: 2965 (phCH, stretching), 1767 (lactamC=O), 1673 (-CONH-), 1608 (-COO-), 1527, 1404, 1316, 1214, 1047 (-SO 3 H), 697cm -1 ;
[0034] 1 HNMR (D 2 O) δ: 1.409 (6H, t-CH 3 ×2), 4.130 (1H, d, -CH-COONa), 4.983 (1H, d, Ar-CH-C=O), 5.446 (2H, m, lactam C-H), 7.442 (5H, m, Ar-H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com