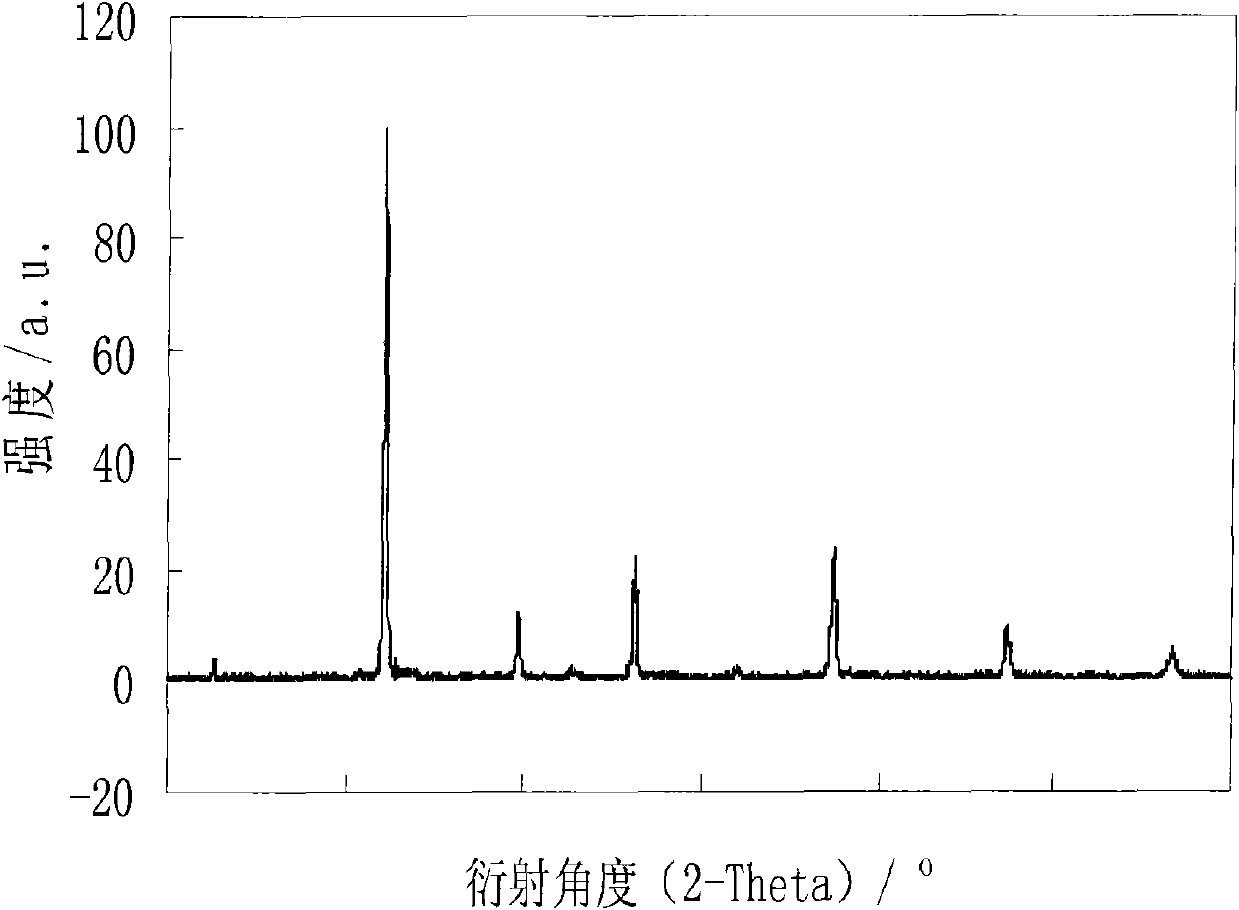
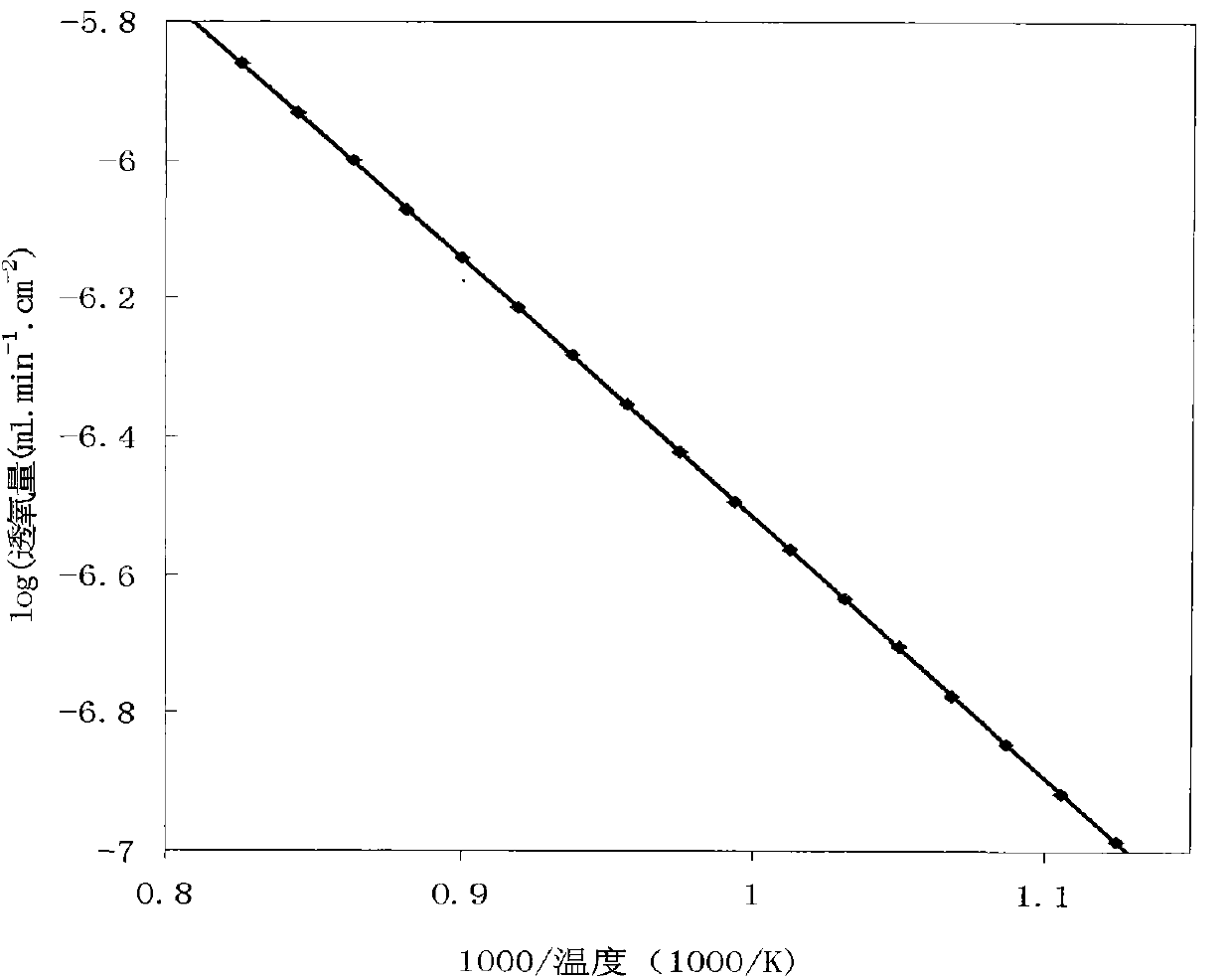
A-site Ba, Sr, Ca ions-doped SrCo0.8Fe0.2O3-delta-base perovskite oxygen permeable membrane material and application thereof
An oxygen-permeable membrane and perovskite technology, which is applied in the field of inorganic oxygen-permeable membrane materials, can solve the problems of difficulty in forming a pure-phase perovskite structure, small bond energy, and difficulty in synthesizing oxygen-permeable membrane materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (I) Ba 0.33 Sr 0.33 Ca 0.34 co 0.8 Fe 0.2 o 3-δ solid phase synthesis
[0028] Weigh the stoichiometric Ba(NO 3 ) 2 , Sr(NO 3 ) 2 , CaCO 3 、Co 3 o 4 , Fe 2 o 3 , according to Ba 0.33 Sr 0.33 Ca 0.34 co 0.8 Fe 0.2 o 3-δ The ratio of the elements to configure the mixture powder, using ethanol as the medium, ball milling in a steel ball mill tank for 5 hours, after mixing evenly, drying in an oven, and then roasting in a muffle furnace at 950 ° C for 5 hours, and then the obtained powder The body was put back into a steel ball mill jar for ball milling for 5 hours, and then roasted, and the ball milling and roasting processes were carried out three times. Then get Ba 0.33 Sr 0.33 Ca 0.34 co 0.8 Fe 0.2 o 3-δ The oxygen-permeable membrane powder is pressed into an oxygen-permeable membrane with a stainless steel mold, and the oxygen-permeable membrane is fired at 1100°C for 5 hours to obtain the required oxygen-permeable membrane.
Embodiment 2
[0030] (II) Ba 0.33 Sr 0.33 Ca 0.34 co 0.8 Fe 0.2 o 3-δ EDTA-citric acid synthesis method
[0031] First, a known concentration of Ba(NO 3 ) 2 Solution is poured in the prepared 2000ml beaker, then add the EDTA acid and ammonia solution of metering ratio, fully stir, make Ba(NO 3 ) 2 Complete dissolution gave a clear solution. Take appropriate amount of CaCO 3 , dissolved with nitric acid to obtain a clear solution with Ba(NO 3 ) 2 The solutions were mixed, and then a proper amount of Sr(NO 3 ) 2 , Co(NO 3 ) 2 , Fe(NO 3 ) 3 The solution is added to the clarified solution prepared above, and the configuration ratio of various metal ions is Ba 0.33 Sr 0.33 Ca 0.34 co 0.8 Fe 0.2 o 3-δ . After the mixed solution of the metal ions used was stirred for a few minutes, an appropriate amount of citric acid and EDTA acid was added to the solution. In the experiment, the ratio of EDTA acid:total amount of metal ions:substance amount of citric acid was 1:1:1.5. T...
Embodiment 3
[0033] (III)Ba 0.4 Sr 0.4 Ca 0.2 co 0.8 Fe 0.2 o 3-δ solid phase synthesis
[0034] Weigh the stoichiometric Ba(NO 3 ) 2 , Sr(NO 3 ) 2 , CaCO 3 、Co 3 o 4 , Fe 2 o 3 , according to Ba 0.4 Sr 0.4 Ca 0.2 co 0.8 Fe 0.2 o 3-δ The ratio of the elements to configure the compound compound, using ethanol as the medium, milling in a steel ball mill tank for 5 hours, after mixing, drying in an oven, and then roasting in a muffle furnace at 950 ° C for 5 hours, and then the obtained powder The body was put back into a steel ball mill jar for ball milling for 5 hours, and then roasted, and the ball milling and roasting processes were carried out three times. Then get Ba 0.33 Sr 0.33 Ca 0.34 co 0.8 Fe 0.2 o 3-δ The oxygen-permeable membrane powder is pressed into an oxygen-permeable membrane with a stainless steel mold, and the oxygen-permeable membrane is fired at 1100°C for 5 hours to obtain the required oxygen-permeable membrane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com