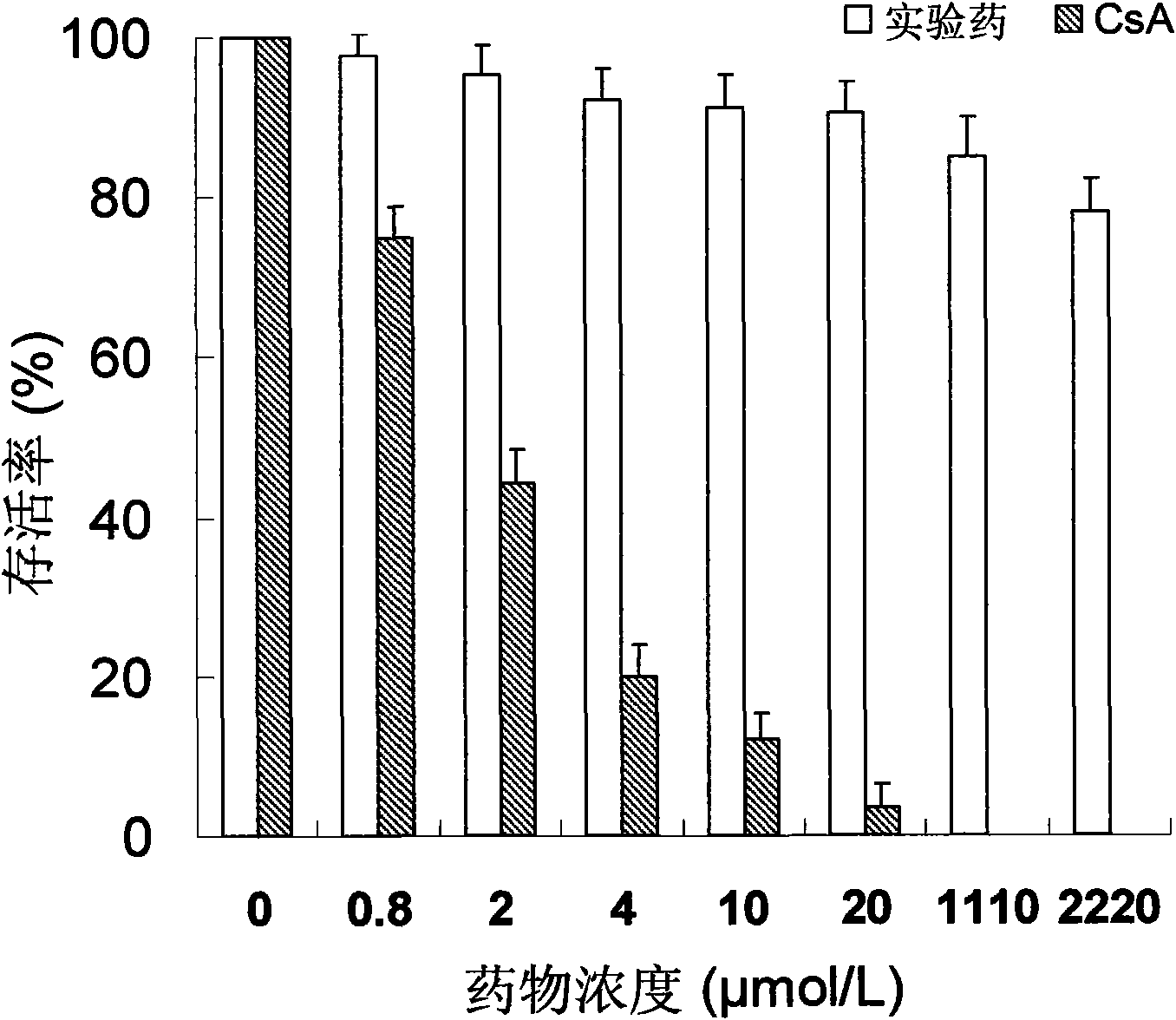
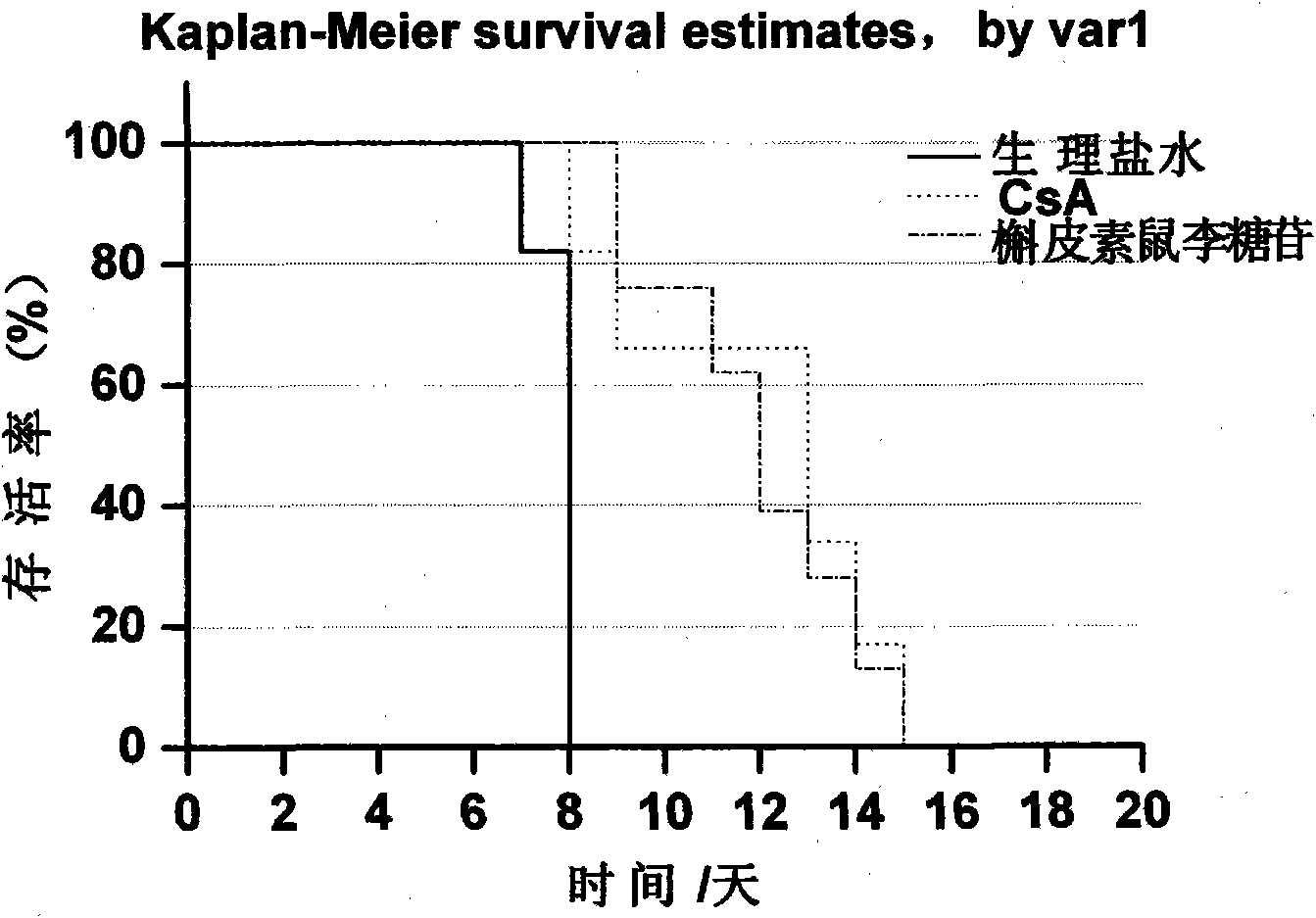
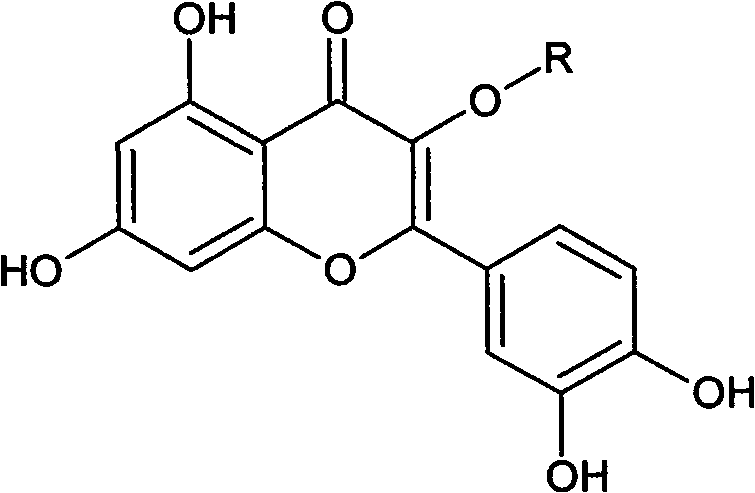
Novel immune suppressor and composition thereof
A technology of immunosuppression and composition, applied in the field of medicine, can solve the problem of no report on the immunosuppressive effect, and achieve the effect of suppressing the rejection of allogeneic organ transplantation, significant immunosuppressive effect, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1. Separation and purification of quercetin rhamnoside
[0039] Take the dried albizia julibrissin and crush it to 10-40 meshes, extract by percolation with 70%-90% ethanol, collect about 10 times the amount of crude drug, and recover under reduced pressure to obtain extract, and then use petroleum ether (bp 60-90 ℃), ethyl acetate, and water-saturated n-butanol for extraction, combined each extract, and reduced pressure to obtain various extracts. Part of the extract from ethyl acetate was subjected to silica gel column chromatography (chloroform:methanol, gradient elution ranging from 100:1 to 0:1), and recrystallized to obtain a yellow powder. The hydrochloric acid-magnesium powder reaction was red, and the Molish reaction was positive. The crystallization was carried out by polyamide thin film chromatography, developed with various developing solvents, and its chromatographic behavior was completely consistent with that of the standard quercetin rhamnos...
Embodiment 2
[0042] Embodiment 2. Quercetin glucoside injection
[0043] prescription:
[0044] Quercetin Glucoside 10g
[0045] Tween 80 8ml
[0046] Ethanol 20ml
[0048] Add water for injection to 100ml
[0049] Take quercetin glucoside, Tween 80, and ethanol and stir to dissolve, and slowly add water for injection containing sodium bisulfite under stirring to obtain a clear solution. Packed into 100 glass ampoules, sterilized after filling. Each injection contains 100mg quercetin glucoside.
[0050] Other quercetins can be obtained with similar prescriptions and processes, including quercetin rhamnoside, quercetin galactoside, quercetin rutinoside, quercetin sambutioside, quercetin diglucoside, quercetin Injection of cortex apirosyl rutinoside, quercetin xyloside, quercetin arabinoside.
Embodiment 3
[0051] Example 3. Quercetin Rhamnoside Tablets
[0052] prescription:
[0053] Quercetin Rhamnoside 10g
[0054] Lactose 100g
[0055] Cornstarch 20g
[0056] Sodium carboxymethyl starch 8g
[0057] Polyvinylpyrrolidone 2g
[0058] Magnesium stearate 0.5g
[0059] Take quercetin rhamnoside and lactose and grind together, pass through a 100-mesh sieve, then mix evenly with corn starch and half the amount of carboxymethyl starch sodium, take 10% polyvinylpyrrolidone aqueous solution to make soft material, pass through a 20-mesh sieve to prepare wet granules , dried at 60°C, granulated with a 20-mesh sieve, mixed evenly with the other half of sodium carboxymethyl starch and magnesium stearate, and punched into tablets with 7mm dimples, with an average tablet weight of 140mg. Each tablet contains 100mg quercetin rhamnoside. The tablet disintegrates rapidly in water, and more than 80% of quercetin rhamnoside is dissolved in water at 37°C within 45 minutes.
[0060] Tablet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com