L-methionine gamma-lyase gene from deep sea metagenome and expression product thereof
A technology of methionine lyase and metagenomics, applied in lyase, genetic engineering, plant gene improvement, etc., can solve the problems of low enzyme yield and difficult scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Acquisition of gene mgl
[0034] 1. Marine sediment metagenomic library construction
[0035] DNA was extracted from marine sediments from four sites in the South China Sea (water depth 1256-2893 m), and DNA from the four sources was combined. Separation by pulsed field electrophoresis and purification by 0.8% agarose gel electrophoresis, the resulting DNA fragment was ligated with the plasmid fosmid and transfected into the host strain E.coli EPI300, spread on LB plate containing 12.5 μg / mL chloramphenicol, 37°C After culturing overnight, 39,600 transformants were obtained, and the clone library was stored at 4°C and -80°C.
[0036] 2. Identification of library clones
[0037] Randomly pick 70 fosmid clones from the above-mentioned deep-sea metagene library and inoculate them in the LB culture solution containing antibiotics, and cultivate overnight at 37°C with shaking. After the culture was concentrated, a plasmid amplification inducer was added, shaken...
Embodiment 2
[0041] Example 2: Protein sequence analysis of gene mgl
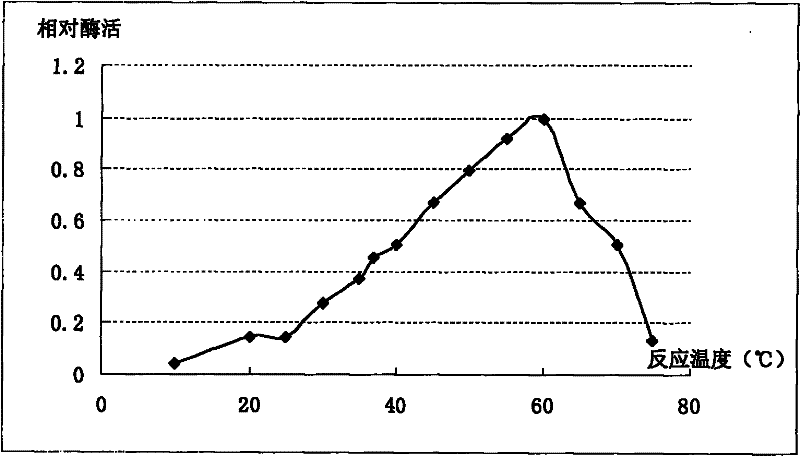
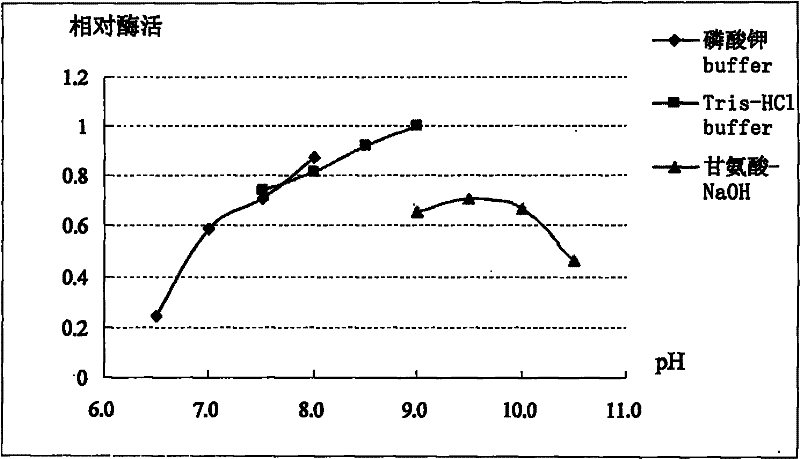
[0042] Using the ExPASy-ProtParam tool of the Swiss Institute of Bioinformatics (SIB), some basic properties of the MGL protein sequence, such as molecular weight, isoelectric point and amino acid composition, were analyzed online ( http: / / www.expasy.ch / tools / protparam.html ). On-line analysis results (see Table 2) show that MGL protein is composed of 426 amino acids, its molecular weight is 46.024kDa, and its theoretical isoelectric point (pI) is 5.28. It is an acidic protein. The half-life in E. coli cells is more than 10 hours, and the half-life in E. coli cells is up to 30 hours. In Table 2, the amino acid sequence of MGL derived from this case is compared with the amino acid sequence of MGL from other sources with the highest similarity, and the results show the obvious differences between the two proteins. Amino acid composition analysis showed (see Table 3) that the protein had higher Ala and Leu contents, fol...
Embodiment 3
[0053] Example three: functional identification of gene mgl
[0054] 1. Cloning of gene mgl
[0055] Design primers for amplifying the full-length gene of mgl according to the known mgl sequence:
[0056] mgl F: 5'-TCAGCGT CCATGG AATCTGATGCG-3' (the underline is the Nco I restriction site)
[0057] mgl R: 5'-CATGAAGTTGTG GAATTC TGTCATG-3' (the underline is the EcoR I restriction site)
[0058] Inoculate the clone f in LB (containing 12.5 μg / mL chloramphenicol), prepare the recombinant plasmid fosmid DNA as a template according to item 2 of Example 1, carry out PCR amplification with the above primers, and use Nco I and EcoR I performs double digestion. At the same time, pET 32a plasmid was prepared, which was also digested with Nco I and EcoRI. The digested products of the two were ligated to construct a recombinant plasmid pET-mgl carrying mgl.
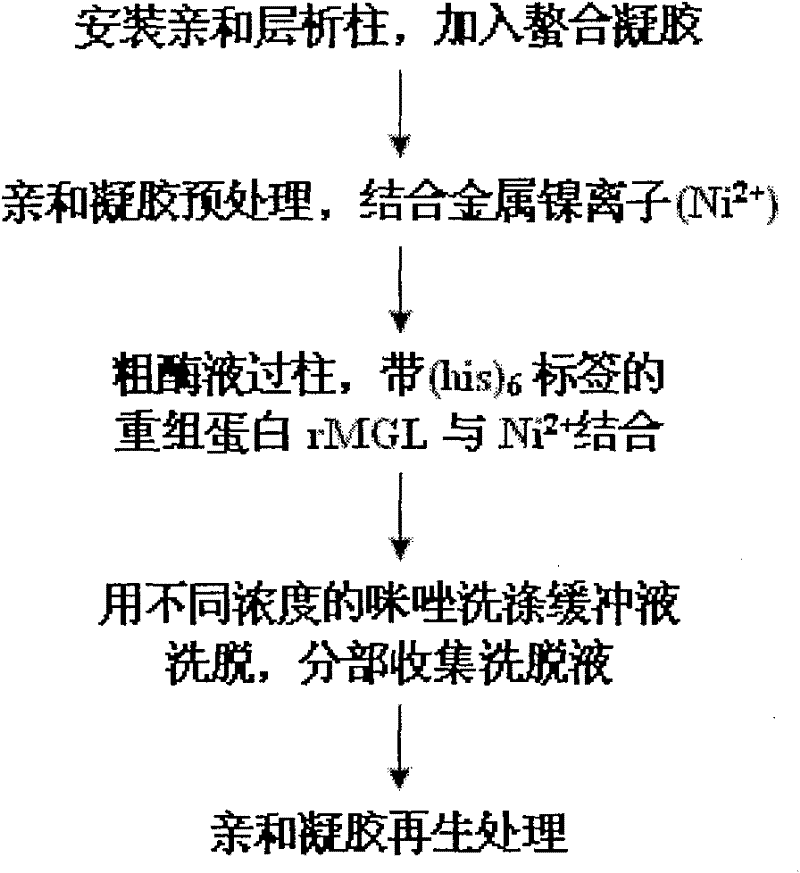
[0059] The recombinant plasmid pET-mgl was transformed into Escherichia coli BL21(DE3), and the recombinant carrying mgl w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com