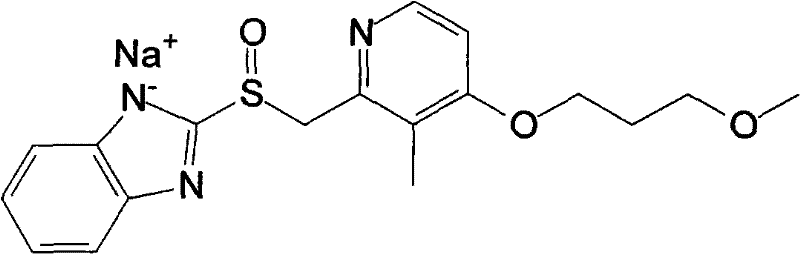
Rabeprazole sodium liposome enteric-coated tablets
A technology of rabeprazole sodium lipid and beprazole sodium lipid, which is applied in the field of medicine, can solve the problems of rabeprazole sodium stability and poor encapsulation efficiency, and achieve discoloration when exposed to heat, increase dissolution rate, Reduce the effect of drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
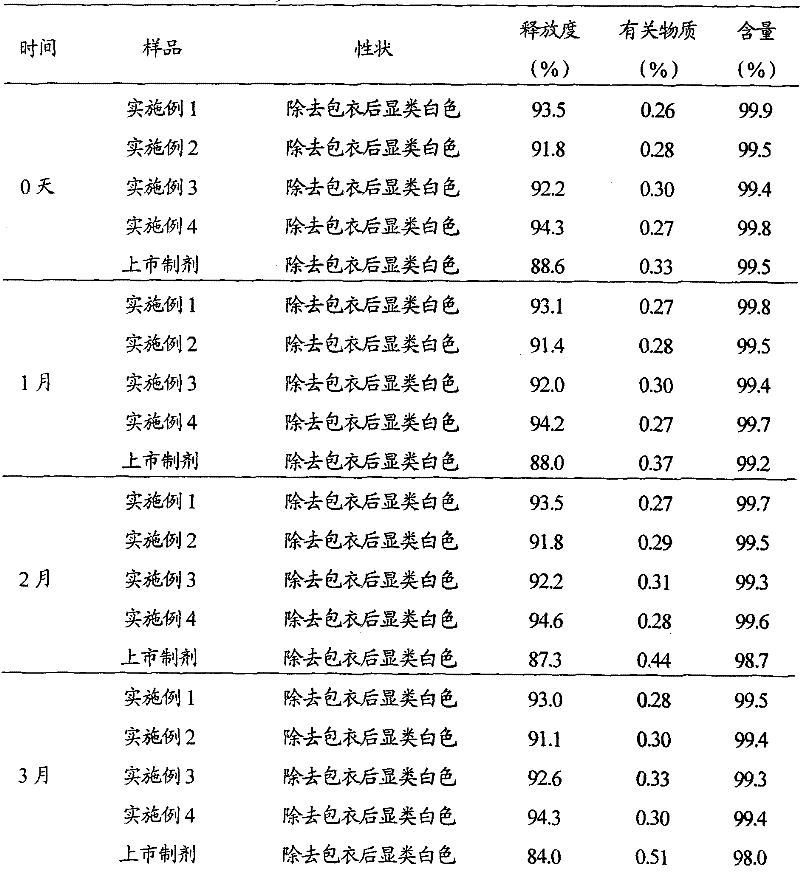
Embodiment 1
[0030] The preparation of embodiment 1 rabeprazole sodium liposome enteric-coated tablet
[0031] Prescription: (1000 tablets)
[0032]Component Dosage
[0033] Rabeprazole Sodium 10g
[0034] Dioleoyl Phosphatidylcholine 40g
[0035] Cholesterol 15g
[0036] Sodium Glycocholate 8g
[0037] Mannitol 70g
[0038] Crospovidone 8g
[0040] Highly substituted hydroxypropyl cellulose 1g
[0043] Povidone K30 1.7g
[0044] Ruitai ES-H Coating Agent 15.8g
[0045] Preparation Process
[0046] (1) Add 40g of dioleoylphosphatidylcholine, 15g of cholesterol and 8g of sodium glycocholate into 600ml of purified water, then add 10g of rabeprazole sodium and mix evenly, heat and stir in a water bath at 90°C for 30min until molten , using a tissue masher to shear and stir 5 times, 5 minutes each time, with a rotating speed of 8000r / min, to obtain the primary emulsion, and then circulate and emul...
Embodiment 2
[0049] The preparation of embodiment 2 rabeprazole sodium liposomal enteric-coated tablets
[0050] Prescription: (1000 tablets)
[0051] Component Content
[0052] Rabeprazole Sodium 20g
[0053] Dioleoyl Phosphatidylcholine 90g
[0054] Cholesterol 50g
[0055] Sodium Glycocholate 30g
[0056] Mannitol 80g
[0057] Crospovidone 10g
[0059] Highly substituted hydroxypropyl cellulose 2g
[0060] Magnesium Stearate 3g
[0062] Povidone K30 4.5g
[0063] Ruitai ES-H Coating Agent 30g
[0064] Preparation Process
[0065] (1) Add 90g of dioleoylphosphatidylcholine, 50g of cholesterol and 30g of sodium glycocholate into 1500ml of purified water, then add 20g of rabeprazole sodium and mix well, heat and stir in a water bath at 70°C for 50min until molten , using a tissue masher to shear and stir 5 times, each time for 10min, and a rotating speed of 5000r / min to obtain the first emulsion, then circulate and emulsify...
Embodiment 3
[0068] The preparation of embodiment 3 rabeprazole sodium liposomal enteric-coated tablets
[0069] Prescription: (1000 tablets)
[0070] Component Content
[0071] Rabeprazole Sodium 10g
[0072] Dioleoyl Phosphatidylcholine 90g
[0073] Cholesterol 50g
[0074] Sodium Glycocholate 30g
[0075] Mannitol 45g
[0076] Lactose 30g
[0077] Low-substituted hydroxypropyl cellulose 15g
[0078] Magnesium hydroxide 8g
[0079] Povidone K30 5g
[0080] Magnesium Stearate 3g
[0082] Povidone K30 4.4g
[0083] Aileyi EOQL55 Coating Agent 28.6g
[0084] Preparation Process
[0085] (1) Add 90g of dioleoylphosphatidylcholine, 50g of cholesterol and 30g of sodium glycocholate into 1500ml of purified water, then add 10g of rabeprazole sodium and mix well, heat and stir in a water bath at 80°C for 40min until molten , using a tissue masher to shear and stir 5 times, each time for 8min, and a rotating speed of 6000r / min to obtain the first emulsio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com