Method for synchronously removing heavy metal and nitrate from drinking water and device thereof
A heavy metal and nitrate technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of low hydrogen utilization rate and low hydrogen solubility, and achieve low ammonia nitrogen and organic carbon content, which is beneficial to ozone. The effect of rapid disinfection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
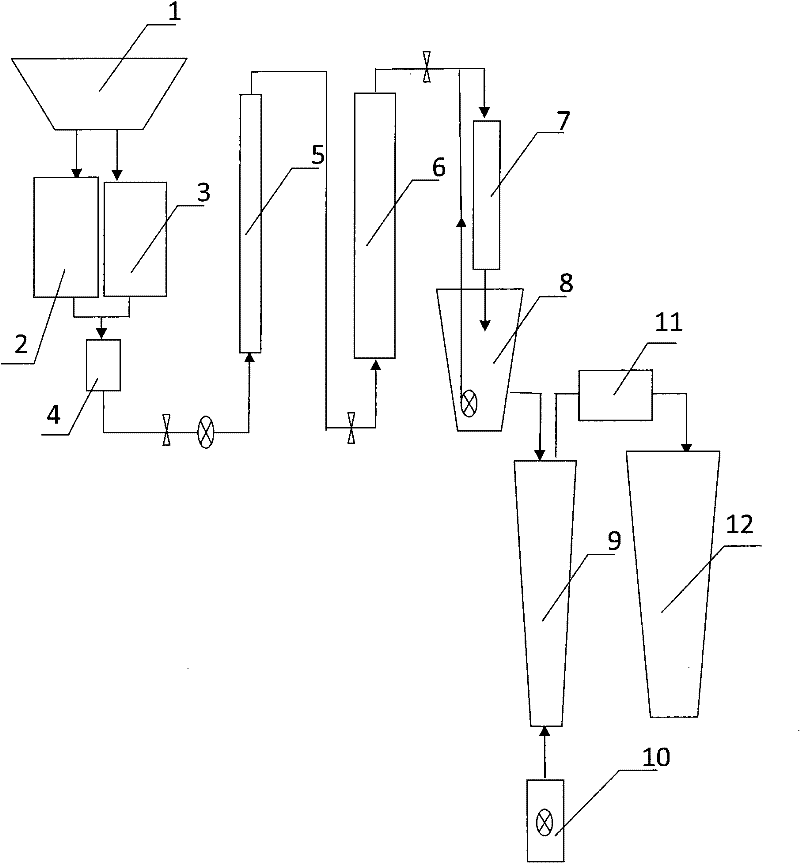
[0029]The metal removal column 5 has a diameter of 10cm and a height of 100cm, and its filler layer is laid five times in the order of quartz sand and iron oxide layer, quartz sand layer, activated carbon and ferrous sulfide layer and quartz sand layer; Layer, quartz sand layer, activated carbon, ferrous sulfide layer and quartz sand layer have a volume ratio of 4:1:4:1; in the quartz sand and iron oxide layer, the volume ratio of quartz sand and iron oxide is 5:1; In the activated carbon and ferrous sulfide layer, the volume ratio of activated carbon to ferrous sulfide is 10:1. The particle size of quartz sand and iron oxide is <1mm, and the particle size of ferrous sulfide and activated carbon is 1-4mm.
[0030] The diameter of denitrification reaction column 6 is 15cm, high 1m, and the filler of its upper half is activated carbon and ferrous sulfide, and volume ratio is 10: 1; The filler of denitrification reaction column lower half is activated carbon and iron oxide, and v...
Embodiment 2
[0040] The difference from Example 1 is:
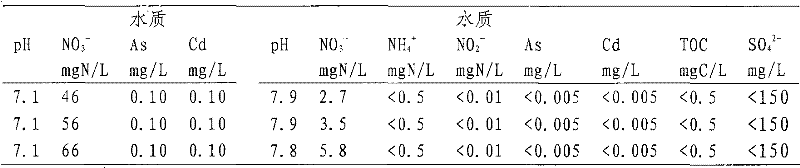
[0041] According to treatment 56mg NO 3 - -80% of the required carbon source in N / L Add formic acid (10mM) and acetic acid (1.5mM) in distribution tank 2, and the effluent from distribution tank 2 and distribution tank 3 will flow into the mixing tank at a ratio of 1:1. The pH of the post water was 4.0. Three kinds of raw water (as shown in Table 2) with nitrate content in the middle and high concentration range were treated sequentially, and other operating conditions were consistent with Example 1. The treatment results are shown in Table 2. For raw water with nitrate content in the range of 46-66mg N / L, the carbon source supply and treatment conditions are the same, but the treatment system can effectively remove NO 3 - , arsenic and cadmium, and NO in the effluent 2 - Compliant with TOC.
[0042] Table 2. Water quality changes before and after treatment of drinking water contaminated by heavy metals and nitrates
[0043] ...
Embodiment 3
[0045] The difference from Example 1 is:
[0046] According to treatment 28mg NO 3 - -80% of the required carbon source amount of N / L is added to the distribution tank 2 with 8mM formic acid, and the outlet water from the distribution tank 2 and the distribution tank 3 flows into the mixing tank at a ratio of 1:1, and the pH of the water after flowing into the mixing tank is 4.9. The three kinds of raw water (as shown in Table 3) with nitrate content in the middle and low concentration range were treated sequentially. The flow rate of the raw water passing through the heavy metal removal column and the denitrification reaction column was 3.6 L / h, and other operating conditions were consistent with those in Example 1. The treatment results are shown in Table 3. For raw water with nitrate content in the range of 18-38mg N / L, the carbon source supply and treatment conditions are the same, but the treatment system can effectively remove NO 3 - , arsenic and cadmium, and NO in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com