Plasma substitute and preparation method thereof
A technology of plasma substitutes and serum albumin, applied in the field of plasma substitutes and its preparation, to achieve the effects of high blood oxygen content, maintenance of blood volume, and improvement of physical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of Lipid Carrier Vesicle Suspension in Oxygenated Plasma Substitute
[0031] Weigh 40 mg of hydrogenated soybean lecithin (HSPC), 1000 mg of polyethylene glycol 1500 (PEG1500), and 100 mg of Tween into a 50 ml beaker, add 10 ml of tert-butanol (analytical grade), and dissolve in a water bath at 60° C. by ultrasonication. After the above substances are completely dissolved and the solution becomes clear, it is divided into 10ml vials with a volume of 2ml per bottle, and it is cooled at 0-4°C for more than 30 minutes. The liquid is milky white and solidified, and it is freeze-dried for 40 hours. The freeze-dried sample was filled with perfluoropropane to replace the air in the bottle, and the bottle was sealed tightly.
[0032] Weigh 300mg of hydroxyethyl starch 130 / 0.4 and 45mg of sodium chloride and dissolve in 5ml of distilled water, inject the stopper into the above vial, mix with the freeze-dried sample, and shake to obtain a lipid vesicle susp...
Embodiment 2
[0033] Example 2: Preparation of Albumin Carrier Vesicle Suspension in Oxygenated Plasma Substitute
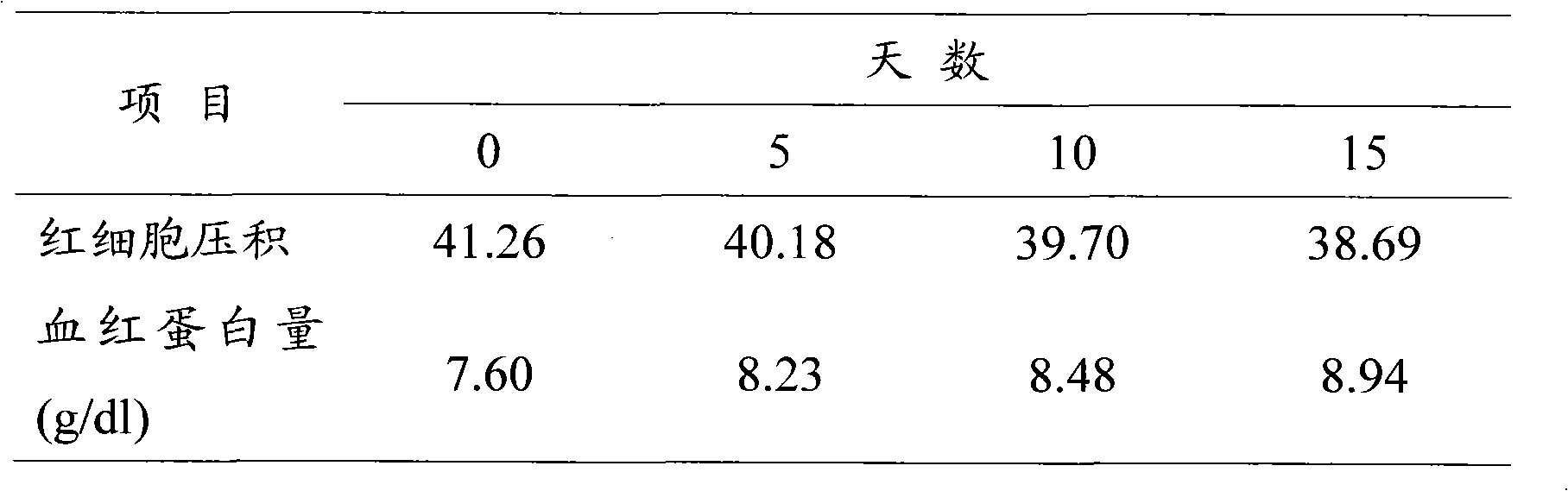
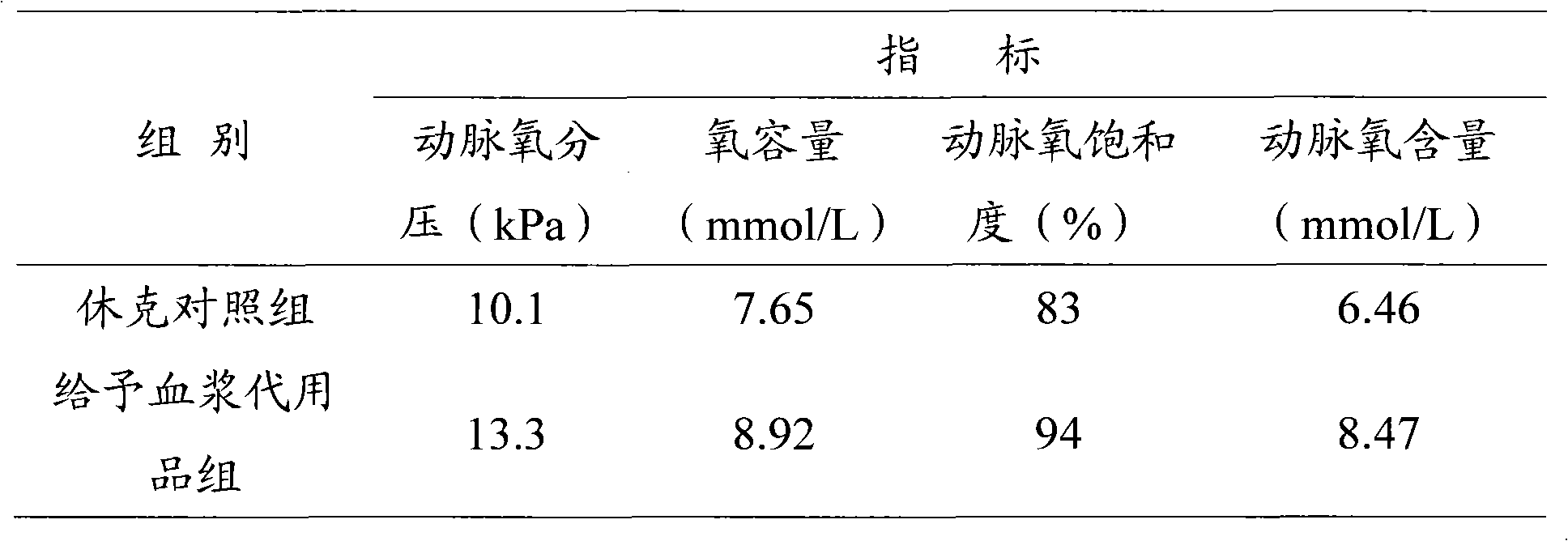
[0034] Weigh 600mg of hydroxyethyl starch 130 / 0.4 and 90mg of sodium chloride and dissolve them in 10ml of distilled water, add 500mg of bovine serum albumin and mix to prepare a 5% bovine serum albumin solution. Continuously ultrasonically oscillate for 60 s, and a large amount of oxygen is introduced from the bottom of the container at the same time. The gas in the bottle was replaced with oxygen, sealed tightly, and stored in a refrigerator at 4°C. The concentration of microbubbles observed under a microscope is greater than 2.5×10 8 Each / ml, the microbubble diameter is between 2-4μm, and the half-life in vivo is 120min.
Embodiment 3
[0035] Example 3: Preparation of Oxygenated Plasma Substitute Carrier Vesicle Suspension by Spray Drying
[0036] Weigh 0.3g of myristyl phosphatidylcholine (DMPC), 0.02g of myristyl phosphatidylethanolamine (DMPE) and 0.05g of poloxamer (188) and dissolve it in 50ml of phosphate buffer, heat to make it a clear solution . 20ml of perfluorohexane liquid was added at a stirring speed of 800rpm to form micron or nanoscale colostrum including perfluorohexane. The formed colostrum was added with an equal volume of 5% hydroxyethyl starch 40 aqueous solution, stirred at a constant speed of 800 rpm to make it fully mixed, and dried by a spray dryer (temperature controlled at 35° C.) to obtain a blank microbubble powder.
[0037] Take 100 mg of spray-dried blank microbubble powder and pack in 5 ml vials, fill with oxygen to replace the air in the vials, and seal them tightly. When using, inject 2ml of normal saline into the septum plug and shake to obtain lipid vesicle suspension. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com