Multiple antigen peptide (MAP) vaccine and application thereof
A vaccine, HLA-A2 technology, applied in medical preparations containing active ingredients, antibody medical ingredients, anti-tumor drugs, etc., can solve the problems of weak immunogenicity, single structure, small molecular weight, etc. The effect of obvious tumor activity and increased number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Design of hTERT dominant T cell epitope MAP vaccine
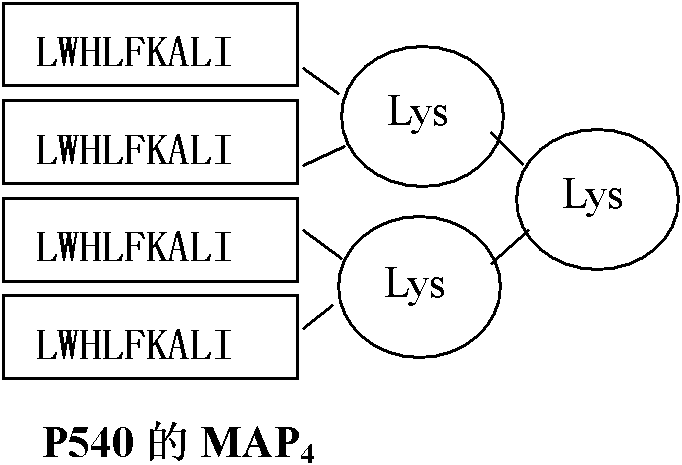
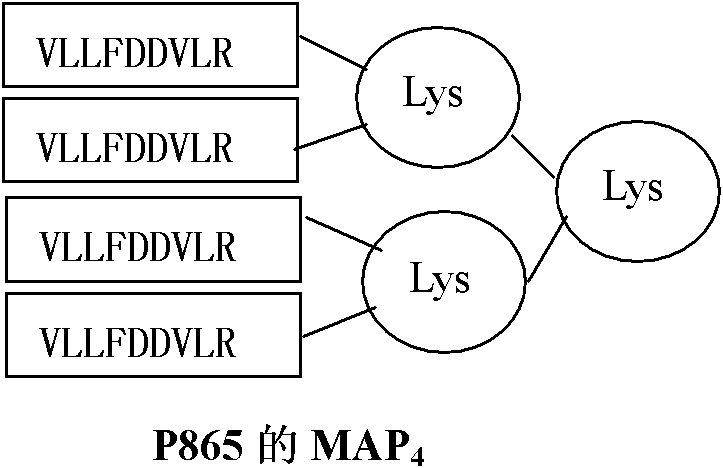
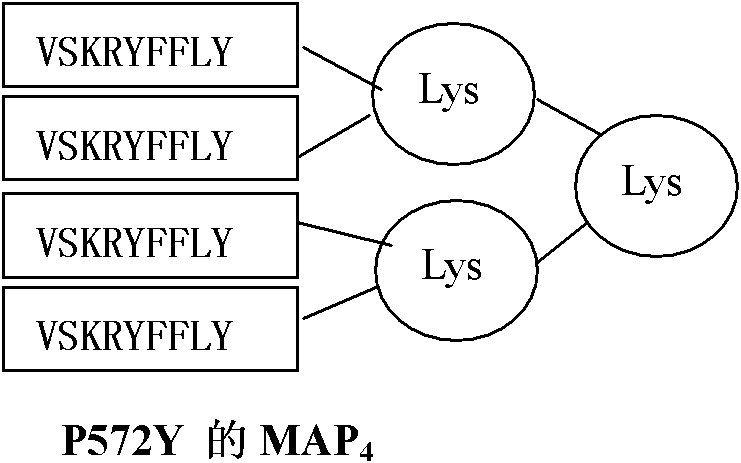
[0058] Since more than 75% of the population is HLA-A2 restricted, the A2 restricted epitope P540 (ILAKFLHWL, Vonderheide RH, Hahn WC, Schultze JL, Nadler LM.The telomerase catalytic subunit is a widely expressed tumor-associated Antigen recognized by cytotoxic T lymphocytes. Immunity 1999; 10:673-679)-SEQ IDNO: 1, P865 (RLVDDFLLV, Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans.Proc Natl Acad Sci USA 2000; 97:4796-4801)-SEQID NO: 2, recessive epitope heteromorphic peptide P572Y (YLFFYRKSV, Scardino A, Gross DA, Alves P, Schultze JL, Graff- Dubois S, Faure O, Tourdot S, Chouaib S, Nadler LM, Lemonnier FA, Vonderheide RH, Cardoso AA, Kosmatopoulos K. HER-2 / neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J. Immunol 2002; 168:5900-5906)-SEQ ID NO: 3 Design its four-branch...
Embodiment 2
[0059] Example 2 Synthesis of hTERT dominant T cell epitope MAP vaccine
[0060] 1. Synthesis and purification of MAP peptide (completed by Shanghai Qiangyao Biotechnology Co., Ltd.): standard Fmoc method was adopted, and arginine was coupled twice; high-pressure liquid chromatography (RP-HPLC) method was used to The molecular weight of the purified polypeptide was determined on an API2000 mass spectrometer; the purified collected solution was evaporated to complete dryness or evaporated to 1-2ml at room temperature under low pressure, and then precipitated with 50ml of ether, collected by a glass sand funnel, and dried in vacuo to obtain Pure peptides were stored at -20°C for later use.
[0061] 2. The identification of MAP peptide: Adopt reverse phase high-pressure liquid chromatography (RP-HPLC) to identify its purity (such as Figure 2-7 shown), using liquid chromatography / mass spectrometry (LC / MS) to identify its molecular weight (such as Figure 8-13 shown).
Embodiment 3
[0062] Example 3 In vitro antitumor activity of human hTERT dominant T cell epitope MAP vaccine
[0063]1. Preparation of human peripheral blood-derived dendritic cells: The mononuclear cells from the peripheral blood of HLA-A2 positive healthy blood donors (provided by the Department of Blood Transfusion, Southwest Hospital, Third Military Medical University) were separated by Ficoll-Hypaque gravity gradient centrifugation. Cells resuspended in RPMI-1640 medium (purchased from Hyclone) containing 10% fetal bovine serum (purchased from Hyclone) were placed in a 37°C incubator for 2 hours, and non-adherent cells were collected and frozen for future use. For the preparation of effector cells later. Adhered cells were further cultured in RPMI-1640 medium containing 10% fetal bovine serum, and recombinant human mononuclear macrophage colony-stimulating factor (rhGM-CSF) and recombinant human interleukin-4 (rhIL-4) were added to it (both purchased from R&D System, Inc., USA), so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com